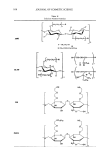
122 JOURNAL OF COSMETIC SCIENCE II( :c C zC 0::: D. E ca: CD CJ ti) ca .,. C CD · .2: e !! , CD 1-.!:,. ·= c 2.0 B � ti 1.5 a.aa i .S 1.0 -� � en.:: e.!! , CD t- .!:.. 0.0 Tyrosinase &-actin 1.2 .----------------, 1.0 + + + 100 200 400 Tyrosinase (564bp) Figure 3. HMF down-regulates tyrosinase gene expression. Bl6Fl0 cells were pretreated with HMF or arbutin for 60 minutes and then stimulated with 1.0 mM theophylline for 72 hours. Cell lysates were analyzed for tyrosinase and 13-actin proteins by Western blotting (A and B). Blots shown are representative of at least three independent studies. Densitometry was performed to quantify the developed bands, and the graphs show the tyrosinase protein content relative to !3-actin. Total cellular RNA was extracted and subjected to RT-PCR analysis of tyrosinase and GAPDH mRNA (C). Stained gels shown are representative of at least three independent studies. M, size marker NC, negative control without RNA sample. The graphs show the tyrosinase mRNA content relative to GAPDH. Data represent mean ± SEM (n = 3). *p 0.05 vs theophylline only. The next experiments examined if HMF could inhibit the tyrosinase-catalyzed melanin formation from the DOPA in situ in the cells. For this purpose, cells were treated with DOPA after pretreatment with HMF or arbutin, in the absence of theophylline. As shown in Figure 4B, incubation of cells with DOPA alone resulted in the accumulation of a dark pigment inside the cells, providing a chance to determine the effect of test materials on the tyrosinase-catalyzed reactions in situ in the cells. As also shown in Figure 4B, the DOPA-dependent cell pigmentation was markedly attenuated by HMF. Arbutin showed a less significant effect, in agreement with the in vitro results. Therefore, it is concluded that HMF can interfere with tyrosinase-catalyzed reactions inside the cells. While genetic background is the most important factor for skin pigmentation (19), other non-genetic factors including hormonal change, chronic inflammation, aging, and ultraviolet light all affect skin pigmentation by stimulating the expression of tyrosinase and other enzymes involved in melanogenesis (2). Tyrosinase inhibitors may be the most non-invasive strategy for the control of skin pigmentation. However, they exhibit less consistent effects in vivo (11-13). The inhibitors of tyrosinase gene expression or matu-
ANTIMELANOGENIC EFFECTS OF HMF 123 A 120 100 cu G) = 80 U) 0 ca ._ C: C: 60 U) 0 0 u L. ,.� 40 0 OH t- - �CH3 HMF e .... 20 · .s 0 0 50 100 200 400 800 1600 Concentration (µM) B --DOPA +DOPA Vehicle HMF 200 µM Arbutin 200 µM Figure 4. HMF inhibits tyrosinase enzyme activity in vitro and in situ in the cells. (A), In vitro tyrosinase activity was determined using B 16 melanoma cell lysates as the enzyme source in the presence of HMF or arbutin at different concentrations. The data are expressed as % of vehicle control and the graph represents mean ± SEM (n = 3). (B) B16F10 cells were pretreated with HMF or arbutin and then supplied with 1.0 mM DOPA or vehicle. Cell images shown are representative of at least three independent studies. ration thus have potential as alternative strategies for the control of melanogenesis (9,10). HMF is one of the naturally occurring furanones currently used in the food industry as a flavor compound (20,21). The present study demonstrates a novel property of HMF, indicating its interference with multiple steps of cellular melanogenesis and supporting its potential use as a depigmenting agent in cosmetics or medicine.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)