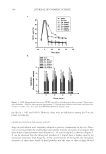
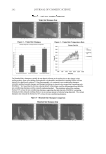
JOURNAL OF COSMETIC SCIENCE 138 the Spi ( p 0.01 and 0.001). However, there were no differences among the P-w, the blank, and the Spi. INHIBITORY EFFECTS OF TYROSINASE ACTIVITY Ange-da and arbutin were originally adopted as positive comparisons in the test. How- ever, it was found that the seaweed had a low ability to resist activation of tyrosinase. The three kinds of pearl powders were diluted to 5, 10, and 20 mg/ml, as shown in Figure 2. It can be observed that the three pearl powders at 5 mg/ml have a higher capacity for tyrosinase resistance than Ange-da. At the concentration of 20 mg/ml, the tyrosinase resistance of these three kinds of pearl powders was the same as that of Ange-da and Figure 1. (A,B) Transepidermal water loss (TEWL) and effect of hydration on skin over time. Values repre- sent the means ± SEM for three separate experiments. ***Signifi cantly different from blank and Spiraea for- mosana, p 0.001. **p 0.01 (one-way ANOVA followed by Scheffe’s test).
COMPARISON OF DIFFERENT PEARL POWDERS 139 arbutin, even reaching a tyrosinase resistance of 90-100%. In comparison, seaweed had a tyrosinase resistance of only 18.7 ± 2.3%. ANTIOXIDANT ACTIVITY Reducing power. The reducing powers of the three kinds of pearl powders compared with ginkgo and Tara are shown in Figure 3A. Gingko and Tara were adopted as positive com- parisons. The three kinds of pearl powders (P-w, P-μ, P-n) have a stronger reducing power with increasing concentration. From the bar diagram, Figure 3B, it can be ob- served that the Tara at 10 mg/ml has a stronger reducing power than ginkgo but that P-μ and P-n have a stronger reducing power nearly equal to that of ginkgo. Meanwhile, at concentrations of 5 mg/ml and 10 mg/ml, P-μ, P-n, ginkgo, and Tara all have a signifi - cantly ( p 0.01) stronger reducing power than P-w. Determination of free-radical scavenging activity by DPPH. Ascorbic acid and ginkgo were adopted as positive comparisons. As shown in Figure 4A, the ability of P-μ and P-n to scavenge the DPPH free radical is observed, whereas P-μ does not increase with increas- ing concentration. Though rather weak, the P-w free-radical scavenging capacity also rises with increasing concentration at 50 mg/ml, its DPPH free-radical scavenging ca- pacity can reach 58.2 ± 0.9%. From the bar diagram in Figure 4B, it can be seen that at 1 mg/ml, the DPPH free-radical scavenging capacities of P-μ and P-n are 33.8 ± 4.6%, and 34.4 ± 6.1%, respectively, which are even stronger than the 24.4 ± 0.2% of ginkgo (positive comparison). At the three different concentrations (1, 5, and 10 mg/ml), except for P-μ at 10 mg/ml, P-μ, P-n, and ginkgo have a distinctly stronger DPPH free-radical scavenging capacity than P-w ( p 0.001). Ferrous ion chelating ability. The three kinds of pearl powders, as well as EDTA for positive comparison and BHA for negative comparison, were diluted to various concentrations according to the above-mentioned method. As shown in Figure 5, the chelating ability of Figure 2. Inhibitory effects of water-soluble pearl powder, ultra-micro pearl powder, and ultra-nano pearl powder on tyrosinase activity with L-tyrosine as a substrate.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)