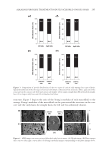
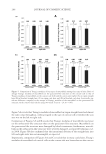
JOURNAL OF COSMETIC SCIENCE 238 Under the conditions employed in the current investigation, the oxidation of L -DOPA by tyrosinase complies with a Michaelis–Menten mechanism. However, since the experiments were carried out in air-saturated aqueous solutions, the Michaelis constant (Km) and the maximum reaction rate (vm) deduced are only apparent. The effect of oxygen concentration on the kinetic parameters needs to be further investigated in subsequent studies. The result illustrated in Figure 5 shows that isoferulic acid is a competitive inhibitor, as increasing the concentration of isoferulic acid resulted in a family of lines with different slopes but sharing a common intercept on the 1/v axis. Figure 2. Effect of isoferulic acid concentration on monophenolase inhibition rate and lag time. Figure 3. Progress curves of L -DOPA oxidation via tyrosinase with isoferulic acid as inhibitor.
EFFECT OF ISOFERULIC ACID ON MUSHROOM TYROSINASE 239 The kinetic parameters of Km and vm for mushroom tyrosinase were calculated from the Lineweaver–Burk plot, using a method introduced by Huang et al. (5). The equilibrium constant KI, in the binding and dissociation equilibrium of the inhibitor and the enzyme, was obtained from the linear plot of Km versus cI, as shown in Figure 6. The inhibition constant of isoferulic acid obtained from the experiment data is also listed in Table I. Figure 4. Effect of concentration of isoferulic acid on diphenolase inhibition rate. Figure 5. Lineweaver–Burk plot of isoferulic acid inhibitory effect on diphenolase activity of tyrosinase.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)