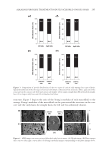
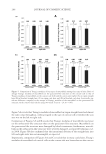
JOURNAL OF COSMETIC SCIENCE 240 Table I Kinetic Parameters of the Isoferulic Acid Inhibitory Effect on Diphenolase Activity cI (mmol/L) Michaelis–Menten equation Km (mmol/L) vm (μM/min) KI (mmol/L) 0 1/v = 0.023/cS + 0.039 0.59 64.5 0.11 0.20 1/v = 0.071/cS + 0.040 1.79 0.40 1/v = 0.111/cS + 0.039 2.84 CONCLUSIONS Described in this paper is a detailed investigation on the inhibition kinetics of isoferulic acid on both monophenolase and diphenolase activities of mushroom tyrosinase. The re- sults showed that isoferulic acid is an effective inhibitor against both monophenolase and diphenolase activities of mushroom tyrosinase. Additionally, the presence of isoferulic acid prolongs the lag period in the inhibited monophenol oxidation. Furthermore, a com- petitive inhibition behavior was observed for isoferulic acid, when it was inhibiting the diphenolase activity of mushroom tyrosinase. Concentrations of isoferulic acid leading to 50% rate inhibition (IC50) on monophenolase and diphenolase activity were calculated to be 0.13 mmol/L and 0.39 mmol/L, respectively, which are much lower than that of arbu- tin (IC50 = 5.3 mmol/L for diphenolase activity). ACKNOWLEDGMENTS This work was fi nancially supported by High Level Teacher Program of Guangdong Province, Science Program of Guangzhou City, and Science Program of Chancheng Dis- trict of Foshan City in Guangdong Province. Dr. Keliang Pang is gratefully thanked for his help in editing this paper. Figure 6. Effect of isoferulic acid concentration on Michaelis constant (Km).
EFFECT OF ISOFERULIC ACID ON MUSHROOM TYROSINASE 241 REFERENCES (1) L.G. Fenoll, M.J. Peñalver, J.N. Rodríguez-López, R. Varón, F. García-Cánovas and J. Tudela, Tyrosinase kinetics: discrimination between two models to explain the oxidation mechanism of monophenol and diphenol substrates, Int. J. Biochem. Cell Biol., 36, 235-246 (2004). (2) I. Kubo, Q.X.Chen and K.I.Nihei, Molecular design of antibrowning agents: antioxidative tyrosinase inhibitors, Food Chem., 81, 241-247 (2003). (3) O. Zarivi, A. Bonfi gli, P. Cesare, F. Amicarelli, G. Pacioni and M. Miranda, FEMS Microbiol. Lett., Truffl e thio-fl avours reversibly inhibit truffl e tyrosinase, 220, 81-88, (2003). (4) H. Huang, X.D. Liu and Q.X.Chen, (title in Chinese: 苯甲醛族化合物抑制蘑菇酪氨酸酶活力的 研究), J. of Xiamen University (Nature Science), 42, 97-101 (2003). (5) C. Tan, W.Y. Zhu and Y. Lu, Aloin, cinnamic acid and sophorcarpidine are potent inhibitors of tyrosi- nase, Chin. Med. J., 115, 1859-1862 (2002). (6) I. Kubo and K.H. Ikuyo, 2-Hydroxy-4-methoxybenzaldehyde: A Potent Tyrosinase Inhibitor from African Medicinal Plants, Planta Med., 65, 19-22 (1999). (7) H. Satooka and I. Kubo, Effects of Thymol on Mushroom Tyrosinase-Catalyzed Melanin Formation, J. Agric. Food Chem., 59, 8908-8914 (2011). (8) O. Nerya, J. Vaya, R. Musa, S. Izrael, R. Ben-Arie, and S. Tamir, Glabrene and Isoliquiritigenin as Tyrosinase Inhibitors from Licorice Roots, J. Agric. Food Chem., 51, 1201-1207 (2003). (9) S.Z. Gong, J. Cheng and Z.R. Yang, Inhibitory Effect of Ferulic Acid on Oxidation of L-DOPA Catalyzed by Mushroom Tyrosinase, Chinese J. of Chem. Engineering, 13, 771-775 (2005). (10) S.Z. Gong, Z.R. Yang and J. Cheng, (title in Chinese: 肉桂酸抑制酪氨酸酶催化反应的动力学研究), J. of Chemical Engineering of Chinese University, 21, 345-349 (2007).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)