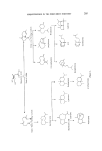
RESPONSE OF THE FROG OLFACTORY SYSTEM 189 Vapour detection system • Ignition ....... i Exhaust '-- .- S'gn [ "- II- .... :-:::--a_ .• Y .... ' ........... .i--•amplifier J _ . 'r. • I ' 500 mL/min. b , I • --1 I ---resetvolt ,50mL/min• • ] • / • L _• Suction regulation PC Flow • -• res trictors ¸--: Zealire / [ pc (L•-: Zeal ire / Flame ionization detector for odorants Pofentlomefric Oscilloscope rec erda r Signal display Figure 5. Diagram of monitoring system for odour stimuli. diameter (od). The flow of sample being withdrawn by this probe needs to be small compared with the whole flow of gases being sampled. On the other hand the sample flow needs to be large enough for low concentrations of vapour to be measured by the fid. A sample flow rate around 2 ml min -x is normally used. The transfer of sample towards the fid is slowed up by adsorption of organics on the inside walls of the probe. In order to minimize this delaying effect, the hydrogen for the flame is also introduced via the probe tube. This is done by means of a smaller tube (0.3 mm od) the end of which is bent and hooked • mm into the open end of the probe. The probe inlet is thus nearly closed and offers resistance to sample flow such that a suction of about 50 cm water gauge needs to be applied to the fid exhaust to maintain this flow. The suction (50 cm water gauge) needs to be adjustable and free of drift and pulsations. This is achieved, as shown in Fig. 5, by means of an adjust- able dip-leg submerged in water and a 3 litre bottle to smooth out pressure pulses. The exhaust from the fid leads downhill to a trap to catch water formed in the flame. The fid itself is lagged to prevent condensation of water vapour inside it, to keep the fid at a steady temperature and to stop heat radiation affecting the frog.
190 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The vapour monitoring system has been used mainly for determining the concentration of odour stimuli delivered by the applicator. Its fast response facilitates the adjustment of the odorant streams so that the desired concentrations are delivered. Once the odorant solutions and flow rates have been adjusted, it is unnecessary always to check the concentrations during a series of olfactory response measurements but only before and after the series. Only when solutions of very volatile substances are used is there any change noticeable in the composition over the period of an experiment. The vapour monitoring system has other important uses. One of these, as mentioned above, was to check the mixing of odorant and carrier streams in the applicator jet. This was done by moving the probe tip 0.2 mm at a time across the 1-mm diameter of the jet orifice with an odorant switched on. The concentration profile so obtained was asymmetrical when mixing was not complete. Incidentally this experiment demonstrated that the sampling system was capable of discerning differences between points only 0.2 mm apart. When the concentration profiles are determined for diameters pro- gressively further and further away from the jet, a three-dimensional picture may be built up showing concentration on the vertical scale and displacement from the jet axis on the horizontal scale. Such a diagram is shown in Fig. 6. This may be the first time that an odorant jet stream has been mapped in this way and it is interesting to note that the concentration has fallen to half its original at a distance 12 mm from the orifice due to the spreading sideways of the odorant. Obviously the positioning of the applicator jet relative to the nasal cavity is of considerable importance. It would be useful to know also how the humidity of the jet streammoriginally saturated--falls off for the same reasons. The olfactory mucus does some- times become dry in spite of the precaution of saturating the carrier gas with water vapour. Another use of the vapour monitoring system has been to check the efficiency of the applicator switching and to obtain some idea of the time course of build-up and fall-off of concentration when an odorant is switched on and off. It was found that, even with much smaller carrier gas flow and increased odorant stream flows, the device reliably and completely switched on and off. Fig. 7 shows the three fid response curves to 2-s applications of acetone, cineole (strong) and cineole 1 000 times more dilute. Ideally the traces should be rectangular. The rounding of the shoulders is due largely to the slow movement of sample up the probe tube
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)