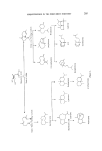
J. Soc. Cosmet. Chem. 25 203-231 (1974)¸ 1974 Society of Cosmetic Chemists of Great Britain Sesquiterpenes industry in the perfumery H. R. ANSARI and A. J. CURTIS* Presented at the 2nd Joint Perfumery Symposium organized by the British Society of Perfumers and the Society of Cosmetic Chemists of Great Britain at Eastbourne on 7-9th May 1973 Synopsis--The developments in SESQUITERPENOID CHEMISTRY are reviewed with especial reference to their application in PERFUMERY. Sesquiterpenes are the group of terpenoids which are formed by the combination of three isoprene units and are found widely distributed in many essential oils in the high boiling fraction. These represent a collection of highly complex and diverse structural systems. Although certain ses- quiterpene-based essential oils, such as oil of sandalwood and vetiver, have been used in perfumery since antiquity, the detailed study of sesquiterpene chemistry commenced only about 20 years ago. This progress has been possible mainly due to modern methods of isolation, structural determi- nation and synthesis. It is generally believed that the future of our industry lies in simulating as many essential oils as possible and hence lessen its dependence on the natural oils. In this respect the detailed and more comprehensive analysis of essential oils is revealing that many commercially important oils contain a number of sesquiterpenes which are important to the overall odour of such oils. Consequently, in many cases the scope of commercially producing synthetic oils will depend on the availability of *Buah Boake Allen Ltd, London El5. 2O3
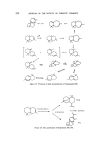
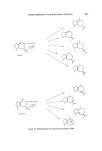
204 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS such sesquiterpenes. Another use of sesquiterpene compounds in per- fumery is to convert the readily available natural sesquiterpenes into various odoriferous compounds on lines similar to monoterpenes. However, the complex nature of this class of compounds coupled with the lack of com- mercially feasible methods of synthesis have prevented a real breakthrough. The increased demand of perfumery materials has pressurized the industry to look for, and accept, synthetic equivalents of the natural products, despite the odour differences between the two. This has led to the commercial production of materials such as geraniol, nerol, citronellol, menthol, citral and the ionones whose isolation from natural oils has since become far less economical. These materials have been produced from heavy organic chemicals (acetylene and acetone) and by transformation of natural monoterpenes which are available in large quantities (tt- and pinene). There is no counterpart of the versatile monoterpene myrcene in sesquiterpene chemistry and an advancement comparable to monoterpenes may appear to be unlikely in the near future. But it is interesting to note that an increasing number of speciality chemicals are already appearing on the perfumery scene. In our opinion many perfumery houses use sesquiter- pene compounds in limited amounts in various formulations which are often closely-guarded commercial secrets. During the last 15 years the industry has had benefit from the extensive research on monoterpenes and perhaps now it is time to explore the ses- quiterpene field with the same seriousness. It is neither the purpose nor the scope of this paper to deal with the systematic description of sesquiterpenes which have been isolated from essential oils and characterized to-date. Instead, we intend to concentrate on the use and chemistry of those ses- quiterpenes and their derivatives which occur in commercially important oils and which have already made an impact on the perfumery industry. Many of these sesquiterpenoid compounds are commercially available. It will be pertinent at this stage to mention that the biogenesis of most sesquiterpenoids in essential oil yielding plants is considered to be based on the cyclization of trans-trans and cis-trans-farnesyl pyrophosphate isomers which in turn are produced from mevalonic acid (1, 2). These biogenetic transformations give rise to some important sesquiterpene groups which will be discussed here (Fig. 1). The acyclic olefins tt- and [3-farnesenes possess interesting odours, which are generally not known to perfumers because of commercial non-availability. The latter compound occurs in many essential oils such as lavender, pepper, hops, copaiba, ylang-ylang and ginger oil (3-5). Peyron, Benezet and
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)