SESQUITERPENES 1N THE PERFUMERY INDUSTRY 205
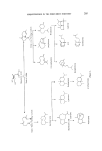
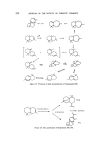
206 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS •-Farnesene Nerolidol ,8-Farnesene Farnesol Figure 2. Garnere (6) found 13-farnesene to be one of the most important constituents of lavender oil. The a-isomer has not been reported to occur in nature but has been synthesized by dehydration of nerolidol or famesol (7, 8). The isomeric alcohols nerolidol and famesol are important perfumery chemicals. Nerolidol was first isolated from neroli oil (9) and since has been found to occur in several other essential oils, e.g. jasmine, citronella, pepper and cabreuva oil (10-12). According to Arctander (13) it has a mild and woody-floral, slightly green odour with excellent tenacity, and good blending and fixative properties. With the availability of synthetic nerolidol, a drastic reduction in its price has been observed. The synthesis which is based on cheap raw materials such as acetylene and acetone, makes nero- lidol a feasible entry into the sesquiterpene field from a commercial view- point (Fig. 3). Several CHECH +CH3COCH:3 steps o *•Na C--CH • Nero Figure 3. The chemical possibilities of converting nerolidol into various sesquiter- penes are enormous. Some work has already been carried out on the iso- merization to famesol (15) and cyclization to an interesting range of cyclci terpenes (16). However, unlike nature, the product selectivity in these cyclizations is low, but further research in this area should prove to be extremely useful.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)