J. Soc. Cosmet. Chem., 28, 717-732 (December 1977) Effectiveness of reduction and oxidation in acid and alkaline permanent waving JANET G. GUMPRECHT,* KANU PATEL, and ROBERT P. BONO, Redken Laboratories, Inc., Biochemical Research Department, 14721 Califa Street, Van Nuys CA 914i 1. Received December 30, 1976. Presented December 6, 1976, SCC Seminar, New York, N. Y. Synopsis A study of the effect of PERMANENT WAVING of HAIR KERATIN was undertaken to more clearly define the chemistry of the reduction and oxidation steps. The processing step was monitored by reacting the thiol groups with ethylene imine to show that 17 to 43 per cent of the cystine is reduced during typical optimal processing times. Hydrogen peroxide and sodium bromate show little differences in effectiveness as oxidizing agents, whereas air oxidation is not effective. Permanent degradation of 10 to 30 per cent of the cystine residues occurred with some cysteic acid being formed during the oxidation reaction. This permanent degradation is reflected in stress-strain properties. Minor amounts of lysinoalanine and lanthionine are produced as well. The major side reaction, however, is the formation of the mixed disulfide .of cysteine and thioglycolate which serves as a diagnostic tool for permed hair. Utilization of a radioactive tag as well as AMINO ACID ANALYSES data have provided evidence that significant amounts of mixed disulfide in permed hair may not only be a result of direct reaction between cysteine and thioglycolate but may also be formed during acid hydrolysis after thiolation of lysine side chain amino groups. This latter synthesis route postulates the formation of a new type of crosslink in permed hair via disulfide formation between cysteine and thiolated lysine. INTRODUCTION The amino acid composition of human virgin hair has been reported by several investi- gators (1-4), but there have been few reports of the chemical effects of permanent waving on human hair, particularly at the amino acid level. Zahn et al. (5) applied sulfur analytical techniques of wool research to hair. Robbins and Kelly (6) showed minor changes in the cystine and cysteic acid content using amino acid analysis, but they did not report side reactions other than the production ofcysteic acid during oxidation. Much discussion of the formation of the mixed disulfide of thioglycolate and cysteine in thioglycolate permanent waved hair has been published. Sch6berl (7) suggested the *Present address: UCLA Bone Research Labs, 1000 Veteran Avenue, Rehabilitation Building, Los Angeles, CA 90024. 717
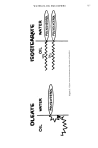
718 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS S(-CH=COS-)xCH = + H=N-PROTEIN H S(-CHCOS-)xCHCONH PROTEIN Figure 1. presence of this mixed disulfide carboxymethylthiol-cysteine (CMT-CySH) in 1953, based on the increased sulfur content of treated hair. Zahn (5) concludes that there is essentially no CMT-CySH under normal waving conditions, but that it is an artifact of hydrolysis. Schulte and coworkers (8) have done some radioisotope studies with la- beled thioglycolate with inconclusive results on the presence of the mixed disulfide in hair. Human and Springell (9) used S a'5 labeled mercaptoacetate to obtain evidence of the formation of the mixed disulfide in wool. Some of the bound label is probably a result of acylation of free amino groups by impurities in the thioglycolate as suggested by White (!0) from experiments with thioglycolate reductions of ribonuclease. These impurities are formed by thiolesterification between the sulfhydryl and carboxyl groups of thioglycolic acid to produce the cyclic dimer, dithioglycolide, or chained polymers of x groups of thioglycolate. These polythioglycolides can result in thiolation of lysine side chains as is shown in Fig. 1. White was unable to show the presence of the mixed disulfide using amino acid analysis when redistilled thioglycolic acid was utilized. However, he did identify mixed disulfide when reagent thioglycolic acid was used. Besides the possible side reaction of CMT-CySH formation in permanent waved hair, there are other suggested degradation products. Horn eta/. (11) have shown that hair can undergo intramolecular rearrangements involving cystine in dilute alkali to yield lanthionine./B-elimination of cystine to yield dehydroalanine can also give rise to the reaction with E-amino groups of lysine to produce lysinoalanine which has been identified in wool by Ziegler (12). This research has been undertaken to reinvestigate the chemical changes in hair keratin during a permanent wave using improved hydrolysis procedures prior to amino acid analysis. Both the reductive and oxidative steps of permanent waving have been moni- tored and sensitive methods including radioisotope tagging have been investigated as aids in elucidating the permanent wave mechanism.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)