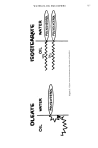
PERMANENT WAVING 721 saturate the hair for 30 min. Then 1.0 ml of Soluene-350 tissue solubilizer was added to each vial which was then placed in a 55øC water bath for 1« hours tightly closed. After cooling, 15.0 ml of Dimilume 30 scintillation fluid was added and the mixture was vortexed. Samples were placed in a Beckman LS-250 scintillation counter and counted one day after equilibration. Counting efficiencies were measured using 50 of C TM toluene as an internal standard. Counting efficiencies ranged from 80 to 88 per cent. From the DPM/mg protein, it was possible to calculate the moles of iodoacetic acid incorporated, and this was divided by the original half-cystine content to give the percentage of half-cystine molecules that were in the reduced state. Controls showed that the original cysteine was negligible. Unlabeled iodoacetic acid was used in parallel experiments and the S-carboxymethyl-cysteine (SCMC) content determined on the amino acid analyzer. S-AMINOETHYLATED KERATEINE Reduced hair (processed only) was alkylated immediately without rinsing, to prevent oxidation, by reacting with excess ethyleneimine under nitrogen atmosphere at room temperature according to the procedure of Cole (15), except that the reaction time was 2« h. This was found as the optimal reaction time for alkylation before degradation of the hair fiber occurs. Amino acid analysis was done on samples before processing, after alkylation, and after the complete perm for comparison. CHEMICALS 1-C TM iodoacetic acid and C TM toluene standard were purchased from New England Nu- clear. • Urea was ultra-pure grade supplied by Schwarz/Mann-•. Soluene-350 tissue solubilizer and Dimilume-30 were from Packard Instruments.+ +. Lysinoalanine was a gift from Jim Vaughn, Durrum Instrument Corp. • Lanthionine and SCMC were purchased from Calbiochem. • SAE-CySH was from Sigma.# All other chemicals were reagent grade. RESULTS Amino acid chromatograms of permanent wave hair hydrolysates always contained a peak between serine and glutamic acid not found in controls or in virgin hair (Fig. 2). This was identified as the mixed disulfide from thioglycolic acid and cysteine by spiking a perm hydrolysate with the synthesized mixed disulfide. The amino acid chromatogram from the reaction of cystine with thioglycolate shows a complexity of products (see Fig. 3). Besides the mixed disulfide, cysteine, and cystine, there are several peaks (I, II, III) with high 440 nm absorption which elute between cysteic acid and aspartic acid. An interesting ninhydrin positive material is also present •New England Nuclear, Boston, MA 02118. q-Schwarz/Mann, Orangeburg, NY 10962. :•Packard Instruments, Downers Grove, IL 60515. •Durrum Instruments Corp., Palo Alto, CA 94303. •Calbiochem, LaJolla, CA 92037. #Sigma Chemical Co., St. Louis, MO 63178.
O• C,d•c & Neutral Am,no A"•ds ydroxylysme ys•ne •st•d•ne i• -- Ammon:a • ,•-Am•no-/J- Guan•d•nopropnomc %• • Arg,n,ne olumn Change • Cyste•c Aod •::• Hydroxyprohne • S-CarboxymethyI-L-Cyste•ne • Asparl•c Ac•d Threon•ne Senne -- Glutam•c Ac•d • •Glyone • Alamne Cysbne • Vahne , Isoleuon•" ............ _, • Leuone •-- •' ' • Norleuone •r• Tyros,ne 1 • -- Phenylalan•n Figure 2. Chromatogram (tracing) of 2.5 nmol of Beckman amino acid calibration mixture with inclusion of peak found in hydrolysates of permanent waved hair. 722
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)