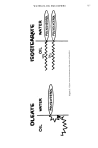
MALODOR FORMATION IN FLUOROCARBON 11 769 z o -I- (a) 7.08 .28 0 e.oo s.7o 20 DRIFT TIME (rn see) Figure 4. (a) Plasmagram of 60/40 propellant 12/11 containing ca. 0.12 per cent CHaNO2 (b) plasmagram of contaminated 60/40 propellant 12/11 containing CHaNO2 (c) plasmagram of DMA. The resultant ions are propelled by an electric field in a counter flowing stream of nitrogen (Fig. 1). The characterization of the contaminants is then made on the basis of drift time and the approximate mass of aggregates of the type [M (H20) ]H +. This method is 3 to 4 orders of magnitude more sensitive than conventional Mass Spec- trometry and is conducted at normal atmospheric pressures. For the present study, methylisocyanide was prepared by a standard procedure (10) from freshly distilled N-methyl formamide and p-toluenesulfonylchloride in quinoline. Vapor samples from contaminated propellant and filters were obtained using gas tight microliter syringes. It was necessary to enclose the filters in plastic bags in order to ob- tain a sufficient amount of sample. Figure 2 shows the plasmagram of the background in clean N2 carrier gas at 204øC. Figure 3(a) shows a plasmagram of vapors from the contaminated filter and Fig. 3(b) a plasmagram from a control sample of pure CHaNC. Both plasmagrams have a pronounced peak at 8.12 •sec. drift time, which is charac- teristic of CHaNC. A standard mixture of 60/40 propellant 12/11 containing CH3NO2 stabilizer gave the plasmagram shown in Fig. 4(a). The peak at 9.70 •sec. was shown to be due to CHaNO2. Comparison of this with the plasmagram of a contaminated sample of propellant (Fig. 4(b)) showed an additional peak at 8.28 •sec., which was identified as
770 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS dimethylamine (DMA) by comparison with a sample of pure DMA (Fig. 4(c)). DMA is a known reduction product of CHaNC (11). B. LABORATORY STUDIES ON CHaNC FORMATION (SEE TABLE I FOR DETAILS) All laboratory studies were run in 3-oz capacity Fisher-Porter Aerosol '• compatibility tubes, type 110-007, fitted with a manifold fitted with an Ashcroft Maxisafe gaugeñ (30 psig vac to 100 psig pressure) and two Whitey• needle valves. Materials that were liquid or solid under ambient conditions were loaded directly into the tube. For ma- terials that were gases under ambient conditions, the tube was placed on the manifold, cooled in a dry ice propellant 11 mixture, and evacuated (oil pump). A cylinder of the desired propellant was connected to the manifold and propellant condensed into the tube. Purging air from the tubes was generally accomplished by heating the tubes in a water bath until an internal pressure of 60 psig was reached and then venting off small amounts of vapor by opening the Whitey valve. Most runs were made with the tube contents agitated by a tinned magnetic stirrer and heated in a water bath using a Thermo-Stir hot plate.# The reaction studies were conducted at two different laboratory sites. Runs 1 to 8 were run in a laboratory where lack of night supervision required shut down of heating every evening and weekend. Furthermore, these reactions were run during the winter. Gauge pressures with P-11 run were often less than zero psig on workday mornings and absolute certainty of anaerobic conditions could not be assumed. All other runs were made where continuous heating was possible throughout the run and positive pressure was assured. Isocyanide formation could only be detected when air was de- liberately admitted to the tubes (Runs 9 to 17). Run 16 was made without purging air so the moisture content may have been slightly greater than the 15 ppm specification of P-11/CHaNO2 blends, but considerably less than the saturation point of 110 ppm in H.,O in P-ll (at 25øC). Run 17 was made with FC-21 which contained 65 ppm P-11. Besides running this experiment in the presence of air, a larger amount of water was added because FC-21 has a greater H20 saturation concentration than P-11. In addition to the above, duplicate sets of runs were made with Propellant 11 contain- ing ca. 0.3 per cent CHaNO2 in pipe bombs fashioned from both corroded and uncor- roded pipe from Propellant 11 service. Runs were made both in the presence and absence of air for one month in ovens held at 60øC. No isocyanide odor was detected. MATERIALS Propellant 11 and methylene chloride were commercial materials and better than 99.8 per cent pure. Fluorocarbon 21 (50 to 500 ppm P-11) was obtained as a byproduct of Propellant 22 manufacture and purified by distillation. It was 99.9 per cent pure by gas liquid chromatography (GLC). Nitromethane** was of 95 per cent minimum purity, 99 per cent minimum nitroalkane. All other reagents were commercial ma- • Fisher-Porter Co., "Lab-Crest" Division, Warminster, PA. •Ashcroft Division of Dresser-Industries, Stratford, CT. •Whitey Co., Cleveland, OH. #Penninsular Manufacturing Co., Gainesville, F1 32602. :x•"• Commercial Solvents Corp., 245 Park Ave., NYC 10017.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)