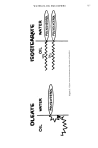
PROTEIN-CHSH + HS-CH:C O-NH-PROTEIN PROTEIN-CH-S-S-CHCON H- PROTEIN Figure 4. tifact produced during hydrolysis in the presence of unremoved thioglycolate. Our lab has found insignificant production of mixed disulfide even during acid hydrolysis of hair in the presence of thioglycolate, so its formation appears to be directly related to the mechanism of perming. The fact that CMT-CySH increases during the oxidative step could arise via two al- ternative routes. Excess thioglycolate present in the hair could react with cysteine in a direct reaction. An interesting mechanism for CMT-CySH formation involves the thio- ladon reaction proposed by White (10) (See Fig. 1). The introduction of this thiol group during processing could result in the formation of a new crosslink during oxida- tion (Fig. 4). If this does occur, one must speculate on whether this crosslink would be better than reformation of a disulfide bond. With the longer arm introduced by thiola- tion, there might be less strain in the hair fiber after oxidation. During acid hydrolysis, this crosslink would be cleaved at the amide bond (Fig. 5) to produce CMT-CySH. The interesting feature derived from White's research is that no mixed disulfide is formed with redistilled thioglycolic acid. This suggests that the thiolation mechanism is an im- portant route for CMT-CySH formation. There are several pieces of evidence that implicate the thiolation mechanism in permanent waving. Our distillation experiments showed less CMT-CySH formation when purer thioglycolic acid was used to formulate wave solutions. Reactions with lysine were greater with decreasing purity, suggesting that there are more polythioglycolides and/or dithioglycolides present to participate in the thiolation with lysine. The radioisotope experiment can best be explained in terms of the thiolation mechanism. There is always higher incorporation of C •4- iodoacetic acid then ac- counted for by reaction of cold iodoacetic acid with cysteine as quantitatively de- termined by the amino acid analyzer. This greater amount of carboxymethylation could easily occur if thiolation introduces additional thiol groups which are available for reac- tion. The radioisotope labeling experiments do not provide an alternate route for de- termination of cystine reduction due to this reaction. However, they could provide in- formation on the extent of thiolation when simultaneous experiments are carried out
730 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS P ROTEl N 'CH' S ' S' CH CON H 'PROTEIN AC I D ,•ROLYSIS C, HCH'S'S'CHCOOH * HN(CH)aC, H COOH CMT 'CYSTEINE COOH Figure 5. to determine the specific labeling of cysteine with the amino acid analyzer. The excess labeling with the radioisotopes should reflect that specific to the thiolated lysine group. In the one experiment set up in this manner, the excess carboxymethylation amounted to 8.2 per cent. For this particular hair sample, this suggests that there are 27 per cent more thiol groups than accounted for by cystine reduction. This suggests that oxidation produces more crosslinks in the hair due to thiolation of lysine side chains. These results are consistent with Sch6berl's (7) report that there is a greater amount of sulfur present in permanent waved hair. The CMT-CySH peak is a diagnostic peak for permanent waving, contrary to reports by Hiragama (17) that it is present in virgin hair. This has implications in the forensic science analysis of hair. In addition to the side reactions that lead to cysteic acid and CMT-CySH formation, there are slight amounts of lanthionine and lysinoalanine formed via /%elimination involving cystine and serine with dehydroalanine as the intermediate (Asquith and Carthew (18) ). These derivatives are found in both acid and alkaline waved hair fibers. The range of cystine reduction necessary for good wave formation has been found to vary over a wide range from 13 to 43 per cent, in these experiments, of the original cystine content. This reduction is related to processing time, but is also indicative of the many parameters involved. These include type of hair, amount of stress during perming, age of perm reagent, operator skills, type of perre, as well as other parameters. Data from numerous experiments point out the difficulty in assigning the quantitative effect of individual parameters. Converting the thiol formed during reduc- tion to a stable derivative using ethyleneimine or iodoacetic acid is a sensitive and ac- curate measure of cystine involvement in perming. Reports by Sanford and Humoller (19) and Inolex Corporation (20) on cystine reduction percentages by reacting with io- doacetic at 100øC in aqueous solution must be carefully scrutinized since these condi- tions could certainly cause cleavage of disulfide bonds.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)