152 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS mental barrier consists of the upper 15-20 •tm of the epidermis. It is the water content of this layer that influences consumer perception of healthy moist skin. In vitro instru- mental methods using nuclear magnetic resonance and infrared spectroscopy (3), differ- ential scanning calorimetry (4), and dielectric (5) and microwave (6) methods have demonstrated the non-homogeneous or triphasic nature of water absorption by the stratum corneum. According to these methodologies, the water content of the stratum corneu. m was found to exist in three different environments: tightly bound from 0.003 to 0.1 gwater/gskin, bound water up to 0.3 to 0.5 gwater/gskin, and free water at higher water contents. The variable nature of water binding has classically been associated with the unique ability of the water molecule to form multiple hydrogen bonds with any combination of its two lone electron pairs and two protons (7). Ideally then, an instrumental method for measuring moisture in the skin should be sensitive to hydrogen bonding, should be correlated to water content, should be nonoc- clusive, fast, and commercially available. Vibrational reflectance spectroscopy in the near-infrared region meets all of the above requirements. It is a photometric technique in which absorbance is directly proportional to concentration. It is sensitive to hydrogen bonding, which causes near-infrared bands to become broader and to shift to lower frequency. State-of-the-art Fourier transform and spinning grating instruments can col- lect optical information with high signal-to-noise ratio in under a minute. Finally, the optical geometry design effectively gathers diffusely reflected light, resulting in a to- tally nonocclusive experiment. This paper will describe the application of a near-infrared reflectance analysis (NIRA) as a probe for both content and bound nature of water in the stratum corneum both in vitro and in vivo. Multiple linear regression techniques will be used to obtain prediction equations for water content from in vitro measurments. Prediction equations from near- infrared using subjective dry leg regression studies (8) as a reference will also be ob- tained using multiple linear regression. NEAR-INFRARED REFLECTANCE ANALYSIS Instruments designed especially for operation in the near-infrared have been commer- cially available since 1971 as a result of the pioneering work of K. Norris (9) and have since been applied to quantitative analyses ranging from moisture in seeds (9) and estimation of body fat composition in vivo (10) to sensory perception of peas (11). These instruments are optically designed with high-energy source and zero-degree illumina- tion optics with 45 ø solid-state detectors in order to optimize collection of diffusely reflected light and ignore spectral scatter (12). This geometry is well suited to quanti- tative analyses since optical data can be transformed to be approximately linear with concentration. The near-infrared region of the spectrum extends from 700 to 2500 nm. The absorption bands that are most prominent in this region are due to overtones and combinations of the fundamental vibrations active in the mid-infrared range (3500- 1500 cm-•) arising from hydrogen covalent bonds. Hydroxyl groups are very strong oscillators and therefore the intensity of their vibrational overtones and combination bands is large in comparison to other covalent bonds. Near-infrared spectral bands are also easily broadened and shifted by hydrogen bonding. This band broadening and band shifting is further compounded in mixtures with a resultant poorly resolved spectrum. Spectral resolution is the determining factor in the choice of quantitative analysis ap- proaches.
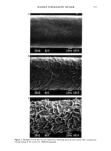
MOISTURE IN SKIN 15 3 ' - TECI4NI(:•::)I• IN .... , =- 4.50 - ? Figure 1. NIR sampling drawer modified to accommodate coils for circulating water bath. Quarter-inch flexible copper tubing was wound into a 4-inch area to accommodate the area of the sampling cup. The simplest spectrophotometric quantitative analyses are performed for the case of complete spectral resolution and absolute spectral band identification. Absorbance can be linearly correlated to an unknown concentration of one analyte at one analytical wavelength according to C = KA• = Klog(l/R0 (1) where A• is the optical absorbance at one wavelength, C is the concentration of the analyte, and R• is the diffuse reflectance at one wavelength. Equation 1 is derived from the Kubelka Munk theory (KM), which is a generalization of the classical Beer Lambert law for media with zero scatter (12). In order to correct for broad band interferences such as baseline drift and particle size effects, equation 1 can be rewritten in its deriva- tive form, C = K(A• - A 2) = Klog(R2/R •) (2) where A1 (log I/R•) is the absorbance at the peak maximum and A 2 (log i/R 2) is the absorbance at baseline. Since NIR spectra are inherently broad and overlapped, identi- fying the wavelength or wavelengths associated with the parameter of interest is usually impossible. The solution is to use the additive Beer's law version of equation 1, which involves absorbances at several wavelengths and takes the form of equation 3:
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)