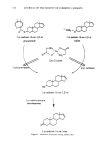
144 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table III Statistical Analysis of Deodorant Effect of Zn-GLY vs Aluminum Chlorhydrate Mean odor score Significance level Application 2 5 hr Zn-GLY 3.12 ACH 3.13 0.945 24 hr Zn-GLY 3.34 0.003 ACH 3.91 Application 3 5 hr Zn-GLY 2.33 ACH 2.95 24 hr Zn-GLY 3.27 ACH 3.88 Application 4 5 hr Zn-GLY 2.60 ACH 2.90 0.005 O.OO3 0.155 24 hr Zn-GLY 3.11 ACH 3.64 0.044 Table I¾ Minimum Inhibitory Concentrations of Zinc Compounds Against Various Bacteria MIC (ppm) Li pop hili c Staphylococcus Staphylococcus Compound diphtheroid epidermis aureus ZnC12 1000 1000 1000 Zn-GLY 1000 1000 1000 Zn-NTA 9600 9600 9600 Zn-HEDPxz 1000 1000 1000 Zn-EDDA 6500 6500 6500 Irgasan 16 0.5 0.5 at the highest concentration of zinc compound. None of the zinc compounds are con- spicuously inhibitory, effective levels ranging from 0.1 to 1%. Zinc glycinate is typical. DISCUSSION Zinc, as zinc glycinate and at the levels tested, binds rapidly to both hair and skin. Specifically, a 100-mM solution deposits approximately 100 }xmol of zinc per gram of tissue. Since this proportion seems to be similar for both skin and hair, we infer that the
ODOR INHIBITION 145 active property being exploited is an affinity for keratin and that skin keratin is similar to hair keratin with respect to the affinity of Zn-GLY. When hair is exposed to such levels of zinc glycinate, the zinc residue left on the hair, even after rinsing with water, is sufficient to completely inhibit both of the bacterial enzymes that were previously implicated in the production of axillary odor (I,3). This conclusion is substantiated by clinical studies that show that 4.5% zinc glycinate is superior to 5% aluminum chlor- hydrate as an underarm deodorant (Table III). Zn-GLY would function as a deodorant probably by preventing the release of com- pounds that contribute significantly to axillary odor. It would appear not merely to block odor receptors or mask unpleasant odor with a strong fragrance. A complex of zinc, in addition to allowing higher zinc concentration, may give rise to a product with longer-lasting deodorancy. We postulate that the complex, acting as a reservoir, may release zinc as the free ion is diluted by eccrine sweat or is consumed by the bacterial enzymes. Although zinc glycinate has been found to inhibit bacterial growth, it is a distinctly poor bacteriostat, i.e., high concentrations are required for its effectiveness (Table IV). We believe that the principal mechanism of axillary odor reduction with this compound is enzyme inhibition. Regardless of the mechanism, the clinical studies discussed here make it clear that zinc glycinate can function as an adequate deodorant. REFERENCES (1) C. Froebe, A. Simone, A. Charig, and E. Eigen, Axillary realodor production: A new mechanism, J. Soc. Cosmet. Chem., 41, 173 (1990). (2) J. N. Labows, K. J. McGrinley, and A. M. Kligman, Perspectives on axillary odor, J. Soc. Cosmet Chem., 34, 193 (1982). (3) E. Eigen, A new mechanism ofaxillary malodor, J. Soc. Cosmet. Chem., 41, 147 (1990). (4) R. R. Marpies, "Effects of Soaps, Germicides, and Disinfectants on the Skin Flora," in The Normal Microbial Flora of Man (Academic Press, New York, 1974), p. 35. (5) J. J. Leyden and R. R. Marpies, Ecological principles and antibiotic therapy in chronic dermatoses, Arch. Dermatol., 107, 208 (1973). (6) J. V. Dubsky and A. Rabas, The formation of salts with glycine, Chem. Abs., 24, 4722 (1931). (7) Hill Top Biolabs, Inc., Cincinnati, Ohio, Private Report to Colgate-Palmolive, 1987. (8) M. Hollander and D. A. Wolfe, Non-Parametric Statistical Methods, Chapter 3 (Wiley, New York, 1973). (9) R. R. Marpies and A. M. Kligman, In-vivo methods for appraising antibacterial agents, TGA Cosmet. J., 1, 26 (1969).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)