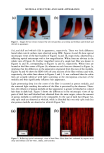
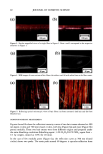
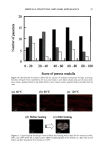
84 JOURNAL OF COSMETIC SCIENCE product residue. Each patient then received the second product according to the ran- domization code and a daily diary to document compliance. After two weeks of product usage every other day, the patients returned their unused second product to the clinic along with their diaries. Their diaries were reviewed for completeness. Each patient was required to complete an evaluation form assessing the product's overall acceptability and efficacy. Gynecological examinations were repeated as described above, including an observation for evidence of product residue. Eva/•atio, form, Each patient completed an evaluation form at the conclusion of each study interval. The evaluation form contained questions designed to assess the patients' opinion of the efficacy of the products. The responses ranged from 1 (very uncomfortable, strongly disagree, or very unsatisfactory) to 7 (very comfortable, strongly agree, or very satisfactory). The results from the two intervals were totaled. The totals were summed to obtain a ranking for each of the questions (Table III and Table IV). STATISTICAL ANALYSIS Residue data were analyzed for differences between groups by means of Fisher's exact test. Comfort scores and agreement scores were analyzed for differences between groups by using a paired T-test. Preference scores were analyzed for differences by using McNemar's test. RESULTS AND DISCUSSION Fifty-one female patients began the study forty-seven patients completed the study. No serious adverse events occurred. Four patients dropped from the study for personal reasons considered unrelated to the test procedures. VAGINAL RESIDUE The grades for vaginal residue following two weeks of product use were significantly (p 0.001) lower for patients using SE vaginal moisturizer compared to patients using Rp vaginal moisturizer (Table lI1). Of the 47 patients completing the study, 41 (87%) were judged to have no residue after using SE vaginal moisturizer for two weeks, while only 25 (53%) patients were judged to have no vaginal residue after using Rp vaginal mositurizer for two weeks (Table lll). After using Rp for two weeks, 17 (36%) patients Table III Scores of Vaginal Residue Residue noted SE vaginal moisturizer Rp vaginal moisturizer None 41 25 Minimal 4 0 Mild 0 5 Moderate 2 12 Significant 0 5 A board-certified gynecologist using a colposcope graded each patient's vaginal area for residue. This table shows the results of his evaluations.
PECTIN-BASED VAGINAL MOISTURIZER 85 received vaginal residue scores of moderate to significant. After those 47 patients used SE for two weeks, only two (4%) patients received moderate-to-significant scores. It can be concluded that pectin-containing SE vaginal moisturizer leaves significantly less vaginal residue than polycarbophil-containing Rp vaginal moisturizer. At the beginning of each study interval, each patient was judged to have no vaginal residue, except for two patients. In each case, the patient first used Rp, and then, after the one-week washout, received a vaginal residue score of 2 at the beginning of her use of SE. One patient's vaginal residue cleared during her two weeks on SE vaginal mois- turizer while the other patient's vaginal residue score increased during her two weeks on SE. This patient was one of the two receiving a vaginal residue score of moderate following two weeks of using SE. This suggested that the one-week washout period allowed for the crossover was insufficient for all patients to clear the polycarbophil residue from Rp. PRODUCT ACCEPTABiLiTY Following the use of each product for two weeks, each patient was required to complete an evaluation form. A quantification of their responses is included in Tables IV and V. Table IV summarizes the responses for both intervals of the study. Each patient re- sponded to each question with a rating from 1 to 7 (1 = very uncomfortable 7 -- very comfortable). The numerical responses were totaled to generate an overall comfort score. Therefore, a higher comfort score indicated higher satisfaction with the product. Al- though SE vaginal moisturizer generally received higher comfort scores, differences were not statistically significant. These data indicated that SE vaginal moisturizer was equiva- lent to Rp vaginal moisturizer in relieving vaginal dryness and in providing vaginal comfort. Table V summarizes the responses for both study intervals. Each patient responded to each statement with a rating from 1 to 7 (1 = strongly disagree 7 = strongly agree). The numerical responses were totaled to generate an overall acceptance score. Therefore, a higher acceptance score indicates stronger agreement with the statement. Although SE vaginal moisturizer generally received higher agreement scores, differences were not statistically significant. These data showed that SE vaginal moisturizer is equivalent to Rp vaginal moisturizer in relieving vaginal dryness and in providing vaginal comfort. Table IV Comfort Scores of Patients SE vaginal Rp vaginal Panelist ratings moisturizer moisturizer Rate your vaginal comfort due to vaginal dryness during the past two weeks 296 278 Rate your vaginal lubrication during intercourse during the past two weeks 293 292 Rate this moisturizer for relieving vaginal dryness 299 295 Rate this moisturizer for providing vaginal comfort 297 294 Rate this moisturizer for overall performance 295 284 The sums of the comfort scores for each patient completing the study are shown for each product. A higher comfort score was considered indicative of a higher degree of satisfaction with the product.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)