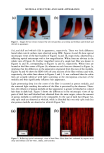
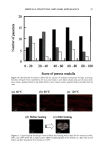
144 JOURNAL OF COSMETIC SCIENCE final concentration) solution in DMSO was used as a positive control in three additional wells. The 96 well plate was incubated at 37 ø for 15 minutes to allow sufficient time for the inhibitor to react. Four ul of 62.5 mM STANA, a substrate for elastase was then added to each well and the plate was incubated for an additional hour. The release ofp-nitroaniline was measured by absorbance at 405 nm. Calculation of elastase activity was as follows: REFERENCE INHIBITOR INHIBITOR BUFFER/SUB- I 2 3 STRATE 4 ELASTASE X X X SUBSTRATE X X X X INHIBITOR (PLANT EXTRACT) X (high conc.) X (low conc.) BUFFER X X X X [Inhibition ] (%) = [conc. ](1) - [conc.] (2) or (3) [conc. pNA] (1) - [ conc.] (4) Inhibited Activity/Normal Activity = % of normal activity. Calculation of amount of inhibition: % inhibition = 100% - % of normal activity. Results and Discussion For test botanical "A", selection of both the solvent and the quantity (w/w) of botanical material to be extracted is important with respect to anti-elastase activity. In the case of test botanical "B", the geographical source of the root is important with respect to anti-elastase activity. Extraction time may be reduced, if higher levels of botanical or higher levels of solvent such as ethanol are used. INHIBITION -1•30TANICAL A • • I • / I • I I a•i References Bicth, J., Spicss, B., and Wcrmuth, C.G. (1974). The synthesis and analytical use of a highly sensitive and convenient substrate for clastasc. Biochemical Medicine 11:350 - 357. Lee, Kun Kook, and Choi, Jung Do, (September/October 1998). Areca catechu L. extract. I. Effects on elastase and aging. J. Cosmetic Science, 49:285 - 297. Lodish, Harvey, et.al., (1995). Molecular Cell Biology. 3ed Edition. W.H. Freeman and Company, New York.
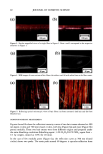
2001 ANNUAL SCIENTIFIC MEETING 145 THE DIFFERENTIAL DISTRIBUTION PATTERNS OF TRANSFERRED MELANOSOMES THE KERATINOCYTE IS REGULATED BY TuE RECIPIENT Id•ERATINOCYTE Ljiljana Minwalla, Ph.D.', Yang Zhao', I. Caroline LePoole, Ph.D. a, R.Randall Wickett, Ph.D? and Raymond E. Boissy, Ph.D.' 'University of CincinnatL Department of Dermatology, Cincinnati, OH •Loyola University of Chicago, Department of Pathology, Maywood, IL and sUniversity of CincinnatL Department of Pharmaceutical Sciences, CincinnatL OH Melanocytes are the cells in the body that are responsible for pigmentation. Melanocytes produce pigment within specialized organelles called melanosomes. In the skin, melanocytes transfer these pigment-filled melanosomes to surrounding keratinocytes. Without this transfer process, pigmentation does not occur. The melanosomes that are found in keratinocytes of Black skin are larger and distributed individually whereas those within keratinocytes of Caucasian skin are smaller and distributed in clusters. This is an important feature that lends itself to differences in complexion coloration. When melanosomes are larger and more spread out, they can absorb more incoming light so that less is refracted. This gives skin a darker coloration. When the melanosomes are smaller and distributed in clusters, they absorb less light and more is refracted thus giving skin a lighter coloration. Figure 1 exhibits the typical differences in melanosome distribution panems that exist between Black and Caucasian skin. Melanosomes within Black keratinocytes (Figure 1 A) are distributed individually throughout the cytoplasm, being predominantly localized apically over the nucleus. In contrast, melanosomes within Caucasian keratinocytes (Figure 1 B) are almost exclusively membrane-bound in clusters but also predominantly localized over the nucleus. Figure I Comparable differences between the distribution ofmelanosomes by histological examination of Black and Caucasian skin. Melanosomes in Black skin (A) are singly distributed throughout the epidermal keratinocytes. Melanosomes in Caucasian skin (B) are found as membrane- bound clusters. (Illustration courtesy of Dr. Raymond E. Boissy).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)