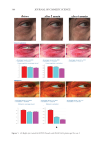
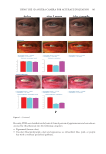
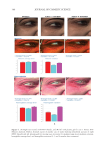
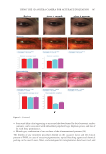
391 J. Cosmet. Sci., 73, 391–403 (November/December 2022) Address all correspondence to Tomohisa Hirobe, tmhirobe@ae.auone-net.jp Mesenchymal Stem Cell-Derived Factors Stimulate the Differentiation of Melanocytes in the Vitiliginous Skin in Combination With Phototherapy TOMOHISA HIROBE AND HISAO ENAMI Shinjuku Skin Clinic, Shinjuku, Tokyo, Japan (T.H., H.E.) Accepted for publication February 1, 2023. Synopsis Although vitiligo treatment is important for cosmetics, full recovery is still difficult. To better improve the efficacy of vitiligo treatment, we selected combined treatment with phototherapy and reconstitution therapy using elastin peptides and umbilical-cord-blood–derived mesenchymal stem cell culture supernatants (UCB- MSCCS). We surveyed whether this treatment can induce repigmentation and/or melanocyte proliferation and differentiation in vitiliginous skin. Punch biopsies of nonlesional, lesional, and treated skin from 51 patients were collected. Three different treatments were (1) monochromatic excimer light (MEL)/narrow-band ultraviolet light B (NB-UVB) (2) MEL/NB-UVB +multilayered treatment (MT) using Dermapen-treated regenerating skin supplemented with elastin peptides and (3) MEL/NB-UVB +multilayered treatment supplemented with key factors derived from stem cell cultures (MTK) consisting of MT and UCB-MSCCS. Although distinct repigmentation failed to be found, melanocyte differentiation and complete restoration of elastin fibers were frequently observed only in the skin treated with MEL/NB-UVB +MTK. Our methods are expected to be useful for developing cosmetics for vitiligo patients. INTRODUCTION Melanocytes differentiate from melanoblasts and produce melanin-laden melanosomes. Melanocytes transport melanosomes to keratinocytes and contribute to the expression of skin color through epidermal melanin pigmentation (1). Vitiligo is prevalent all over the world and its incidence is 0.1–2% of humans. Vitiligo is characterized by white patches caused by deficiency or dysfunction of epidermal melanocytes (2). Vitiliginous skin is serious cosmetically and socially, because white patches are present within visible skin areas such as the face, neck, and hands. Moreover, treatment outcomes are still cosmetically unsatisfactory. Monochromatic excimer light (MEL, 308 nm) and narrow-band ultraviolet light B (NB-UVB, 311 nm) have been used for one of the effective treatment modalities that can induce repigmentation (3,4). However, the treatment requires a long time and its efficacy is unsatisfactory. Skin wounding is also effective for inducing repigmentation (1). Numerous growth factors and cytokines are released from keratinocytes and fibroblasts during wound
392 JOURNAL OF COSMETIC SCIENCE healing (1). It is possible that these factors induce the differentiation of melanocytes in vitiliginous skin. Moreover, our previous study showed that elastin fibers, but not collagen fibers, dramatically decreased in the vitiliginous skin (5), suggesting that recovery of elastin fibers may induce melanocyte differentiation in the vitiliginous skin. Ferrous ferric chloride (FFC) controls oxidation and reduction and stimulates the proliferation and differentiation of human melanocytes, keratinocytes, and fibroblasts (6), suggesting that FFC may stimulate melanocyte differentiation in vitiliginous skin. Moreover, mesenchymal stem cells (MSCs) have been reported to suppress immune reactions, decrease levels of inflammatory cytokines (7), release numerous growth factors that stimulate the proliferation and differentiation of neighboring cells (8), and stimulate the proliferation of melanocytes when cocultured with melanocytes (9). To better obtain successful treatments for vitiligo, we investigated whether combined treatment of MEL/NB-UVB exposures, multilayered treatment (MT) using regenerating skin after making numerous fine holes by Dermapen followed by the addition of collagen/ elastin peptides and FFC, and multilayered treatment supplemented with key factors derived from stem cell cultures (MTK, MT +culture supernatant of MSCs) can induce elastin fibers’ restoration, melanocyte differentiation, and repigmentation. These trials for the vitiligo treatments are expected to develop new and powerful cosmetics that can be useful for vitiligo treatments. MATERIALS AND METHODS COLLECTION OF SKIN SAMPLES Fifty-one vitiligo patients (4–79 years old, 23 females and 28 males) who possessed white macules in many skin sites visited our clinic from September 2018 to January 2021 (Table I). All were nonsegmental, stable vitiligo patients. Forty-eight patients are Japanese, two are Chinese, and one is Middle Eastern. Before starting, we explained the methods of MEL/NB-UVB therapy and skin wounding/sampling and obtained informed consent. We started exposures of two minimal erythema doses of MEL or NB-UVB. Only lesional areas were exposed to MEL/NB-UVB. The patients were divided into three groups: 1) MEL/ NB-UVB alone 2) MEL/NB-UVB +MT and 3) MEL/NB-UVB +MTK. Numerous fine holes (1.5 mm in depth) were made on lesional skin by Dermapen 4 (DermapenWorld, Terrey Hills, Australia), and a solution (1 ml) for MT consisting of collagen peptide (0.19 mg/ml), elastin peptide (9 mg/ml), and FFC (0.001 ng/ml) was quickly added. A solution (1 ml) for MTK in which freeze-dried umbilical-cord-blood- derived mesenchymal stem cell culture supernatants (UCB-MSCCS) were dissolved in MT solution was similarly added. Then the treated skin was covered with adhesive tapes and rolled with bandages. Five days after this treatment, MEL or NB-UVB was continuously exposed on the treated skin two to three sessions per week. Five skin areas were fixed: 1) nonlesional (more than 2 cm apart from the edge of white macules) 2) lesional 3) (MEL/NB-UVB)-treated 4) (MEL/NB-UVB +MT)-treated and 5) (MEL/NB-UVB +MTK)-treated. Areas four and five were pretreated with Dermapen, but not area three. In our preliminary study, Dermapen alone (area three) failed to induce the differentiation of melanocytes and melanoblasts and repigmentation and to increase the density and thickness of elastin fibers. Three punch biopsies (0.6–1.3 mm in diameter,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)