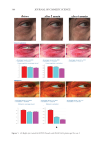
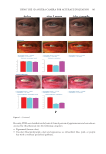
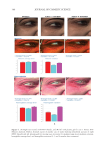
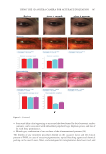
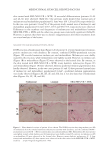
401 MESENCHYMAL STEM CELL-DERIVED FACTORS melanoblast differentiation and recovery of elastin fibers. These results suggest that MEL/ NB-UVB exposures cannot induce melanocyte/melanoblast differentiation, but they can promote melanocyte/melanoblast differentiation-inducing effects of MTK. Hachiya et al. (12) reported that stem cell factor was increased in keratinocytes after exposures of UVB (305 nm). Stem cell factor might be one of the promoting factors that can help the differentiation-stimulating effects of MTK toward melanocytes and melanoblasts. In the present study, even in the recovered epidermis that possessed a normal size of melanocyte population, epidermal melanin pigmentation was poor. Formations of melanosomes and dendrites in melanocytes as well as the transport of mature melanosomes to surrounding keratinocytes may need a long time. Epidermal melanin pigmentation seems to be greatly increased only after active and continuous transport of numerous mature melanosomes to keratinocytes. Therefore, it is reasonable to think that the skin possessing melanocytes and melanoblasts induced by the treatment with MEL/NB-UVB +MTK requires a long time to establish obvious epidermal melanin pigmentation. UCB-MSCCS is known to contain numerous growth factors such as basic fibroblast growth factor, hepatocyte growth factor, and vascular endothelial growth factor (13). These growth factors are also known to induce the proliferation and differentiation of melanocytes (14,15). The combination of these growth factors present in UCB-MSCCS may contribute to the induction of melanocyte differentiation in cooperation with a MEL/NB-UVB–derived factor such as SCF (12). Moreover, these growth factors are known to stimulate the proliferation of fibroblasts (16). Increased fibroblast proliferation may elicit an increase in elastin synthesis. It has been reported that dermal melanocytes are intimately connected with elastin fibers (17). Moreover, normal mouse melanoblasts expressed elastin-binding protein, and elastin- derived hexapeptide stimulates melanin synthesis and dendricity of melanocytes (18), suggesting that the interaction between melanoblasts and elastin-binding protein is started early in the development of mice. In normal human skin, elastin peptides in the presence of FFC also stimulated the proliferation and differentiation, DOPA reactivity, melanogenesis, and dendritogenesis of epidermal melanocytes (19). In addition to these previous reports, the present findings that elastin fibers in the dermis treated with MEL/NB-UVB +MTK were increased and reached the basement membrane of the epidermis may suggest that the interaction between elastin fibers and epidermal melanocytes/melanoblasts is indispensable for the differentiation of melanocytes in the vitiliginous skin. The present results that the recovery of elastin fibers in the treated lesions with MEL/ NB-UVB +MT compared with MEL/NB-UVB +MTK was greatly reduced may suggest that UCB-MSCCS contains upregulating factors toward elastin fiber development. It remains to be studied in a future study what substances included in UCB-MSCCS are involved in upregulating elastin fiber production. Our future study may be expected to contribute to the development of new cosmetics that are effective for treating vitiligo development. CONCLUSION MTK supplemented with UCB-MSCCS in combination with MEL/NB-UVB exposures may be one of the useful treatment modalities to recover vitiligo within a relatively short time. Taken together, our methods are expected to be useful for developing new cosmetics for vitiligo patients and new vitiligo treatments. Our treatments are thought to be permanent ones, since we observed that the melanocytes in the treated lesions remained
402 JOURNAL OF COSMETIC SCIENCE active after cessation of the treatments. We observed the activity of the treated melanocytes 6 months after treatment cessation. ACKNOWLEDGMENTS The authors thank the staff of Shinjuku Skin Clinic for their helpful technical supports. REFERENCES (1) T. Hirobe, How are proliferation and differentiation of melanocytes regulated? Pigment Cell Melanoma Res., 24(3), 462–478 (2011). (2) C. Bergqvist and K. Ezzedine, Vitiligo: A review, Dermatology, 236(6), 571–592 (2020). (3) K. K. Park, W. Liao, and J. E. Murase, A review of monochromatic excimer light in vitiligo, Br. J. Dermatol., 167(3), 468–478 (2012). (4) R. Li, M. Qiao, X. Wang, X. Zhao, and Q. Sun, Effect of narrow band ultraviolet B phototherapy as monotherapy or combination therapy for vitiligo: A meta-analysis, Photodermatol. Photoimmunol. Photomed., 33(1), 22–31 (2017). (5) T. Hirobe, H. Enami, and A. Nakayama, Elastin fiber but not collagen fiber is decreased dramatically in the dermis of vitiligo patients, Int. J. Dermatol., 59(10), e369–e372 (2020). (6) T. Hirobe, Ferrous ferric chloride stimulates the proliferation of human skin keratinocytes, melanocytes, and fibroblasts in culture, J. Health Sci., 55(3), 447–455, (2009). (7) J. Galipeau and L. Sensebe, Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities, Cell Stem Cell, 22(6), 824–833 (2018). (8) A. Tyndall and F. A. Houssiau, Mesenchymal stem cells in the treatment of autoimmune diseases, Ann. Rheum. Dis., 69(8), 1413–1414 (2010). (9) L. Zhu, X. Lin, L. Zhi, Y. Fang, K. Lin, K. Li, and L. Wu, Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through PTEN pathway in vitiligo, Stem Cell Res. Ther., 11(1), 26, (2020). (10) T. Hirobe and H. Enami, Excellent color-matched repigmentation of human vitiligo can be obtained by mini-punch grafting using a machine in combination with ultraviolet therapy, Dermatol. Sinica, 36(4), 203–206, (2018). (11) T. Hirobe and H. Enami, Histochemical study of the distribution of epidermal melanoblasts and melanocytes in Asian human skin, Skin Res. Technol., 25(3), 299–304 (2019). (12) A. Hachiya, A. Kobayashi, A. Ohuchi, Y. Takema, and G. Imokawa, The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation, J. Invest. Dermatol., 116(4), 578–586 (2001). (13) B. R. Weil, A. M. Abarbanell, J. L. Herrmann, Y. Wang, and D. R. Meldrum, High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation, Am. J. Physiol. Regul. Integr. Comp. Physiol., 296(6), R1735– R1743 (2009). (14)Y .Yamaguchi, S. Itami, H. Watabe, K. I. Yasumoto, Z. A. Abdel-Malek, T. Kubo, F. Rouzaud, A. Tanemura, K. Yoshikawa, and V. J. Hearing, Mesenchymal–epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation, J. Cell Biol., 165(2), 275–285 (2004). (15) T. Hirobe, Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes, Pigment Cell Res., 18(1), 2–12 (2005).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)