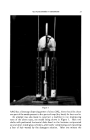
ALKYLOLAMIDES IN SHAMPOOS 33 • . •.. •..• .... .. ., •:•-•'.': :...-:•.,•, •. . ..•... . -. , ,. ::.-: .. . ..... '.:.•' ,, -- . . .: .• ............. :::- -. ....•.•.•.:..... •,:..•.....,...,:, •.•'". .. ,... ,:.'• .. .. ':•' --.•.'--. • .... .. -.. Figure 1 AA62 has a limesoap dispersing power of about 20•, there should be about one part of the amide present to five parts of soap (dry basis) for best results. An attempt was also made to construct a machine to run shampooing tests of the above type, one model being shown in Figure 1. Here two shafts with perforated horizontal disks fixed to the bottoms reciprocated up and down inside glass cylinders, alternately compressing and expanding .a bun of hair wetted by the detergent solution. After one minute the
34 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS machine was stopped, the lather was floated out of the hair by addition of water, and its volume measured. Unfortunately, reproducibility was not entirely satisfactory, although this might be improved by suitable design changes. Many cosmetic chemists attempt to screen shampoos by shaking dilute solutions in graduates, and a few tests were therefore carried out to check this method. For example, a 0.3% solution of the lauryl sulfate was com- pared with a 0.3% solution containing a 1: 1 blend of alkylaryl sulfonate and Ninol AA62. In the shampoo test the blend had been found superior. In both cases hard water was used and 0.3% of the mineral oil-lanolin blend was added to simulate the shampooing of oily hair in hard water. On shaking 20 ml. of each solution in a 100-mi. graduate, the lauryl sulfate gave 40 ml. of foam, the blend 30 ml. This is the reverse of the shampoo tests. The shake test was then repeated, but this time at the 3.0% con- centration level which was actually used in the shampoo tests. The foam volumes were now found to be 48 ml. for the lauryl sulfate and $$ml. for the blend,' which is at least in qualitative agreement with the shampooing results. In general, however, the shake test has not been found very reliable as a measure of lathering power on the hair. VISCOSITY A moderately high viscosity has been found desirable in liquid shampoo products, partly for sales appeal and partly because a certain amount of body will help prevent the liquid from running off the head and into the eyes. In many cases thickening has been achieved by water-soluble gums, colloidal silicates or even salt additions, but it is more logical to use deter- gents for this purpose, if possible, rather than inert materials. Hence the unique thickening action of the alkylolamide type detergents has long been an important reason for their use in shampoos. Viscosity can be measured in many different ways, some of which are illustrated diagrammatically in Figure 2. In the current study, a torque- type instrument known as the Brookfield Viscosimeter was employed and is shown diagrammatically in Figure 3. Viscosity-concentration curves for several different Ninol alkylolamides in distilled water are shown in Figure 4. As can be seen, the alkylolamides give viscous solutions even at con- centrations as low as 2 per cent (for comparison it might be mentioned that Nujol has a viscosity of about 90 centipoises). Anionic detergents, on the other hand, usually remain thin up to 20 or 30 per cent concentration. Addition of anionic detergents to alkylolamide solutions tends to de- crease their 7iscosity, hence somewhat greater amounts of the amides are required to thicken lauryl sulfate or alkylaryl sulfonate shampoos than to thicken water. The viscosities of such blends are shown in Figures 5 and 6, where the total active detergent in solution is maintained at 15% in all
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)