DETERMINATION OF TRACE COMPONENTS IN DYE 557 per cent diethylaminophenol as shown in the table. A linear relationship is ob- tained. The average error (deviation from the mean) is 1.5%. Similarly, between 0.2 and 0.6% phthalic acid were added to several sam- ples of Rhodamine B stearate and assayed. The results obtained are given in Table II. Linearity of response is likewise obtained. The error is somewhat greater, primarily at the 0.2% level. Table 11 Reproducibility of Phthalic Acid, Glc Response Phthalic Acid Deviation from (%) Peak Height/% Mean (%) 0.6 6.15 3.9 0.6 6.10 3.0 0.4 5.99. 0.0 0.4 6.23 5.2 0.2 5.20 12.2 •-= 5.92 •: 4.9 Silylation is an equilibrium reaction. Phthalic acid and m-diethylamino- phenol compete for the trimethylsilyl group with a vast excess of rhodamine and stearic acid. Tri-Sil/BSA is a solution of N,O-bis (trimethylsilyl) acet- amide and a catalyst in dimethylformamide. If a sufficient amount of this re- agent is added to drive all of the reactions to completion, the dilution of the m-diethylaminophenol and phthali½ acid is too great for quantitation. The bulk of the solvent is still being eluted at the retention time .of these sub- stances. If N,O-bis(trimethylsilyl) acetamide is used alone as the sfiylating agent, the dye is insufficiently soluble in it and the absence of the catalyst results in a nonlinear response. By proper balancing of sample size and N,O-bis (trimeth- ylsilyl) acetamide Tri-Sil/BSA concentrations we achieve conditions under which we obtain linearity of response which extrapolates to zero at zero con- centration of these impurities. In view of the equilibria involved, for maxi- mum accuracy it is preferable to use standards closer to the sample concen- tration if the samples fall much above or below the concentrations in the cali- bration curves i.e., 0.08%-0.32% for m-diethylaminophenol and 0.2%-0.6% for phthalic acid. o-(2-Hydroxy-4-diethylaminobenzoyl) benzoic acid (HD BA) From 0.3-0.7% HDBA were added to aliquots of Rhodamine B stearate, which were then assayed by tlc as described above. The results, given in Table III, demonstrate linearity of response and satisfactory reproducibility. A preliminary study has shown that HDBA can also be separated from the other components by high-temperature glc. A 200-mg sample of the dye in
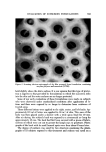
558 JOURNAL OF THE SOCIETY OF COSMETIC CItEMISTS Table III Recovery of Added HDBA HDBA Absorbance/% Deviation from (%) Mean (%) 0.3 0.840 2.7 0.4 0.897 3.9 0.5 0.837 3.0 0.6 0.880 2.O 0.7 0.863 0.0 •-- 0.863 •--- 2.3 the presence and absence of 1 mg of HDBA was sililated by heating at 70øC for 15 rain with 1 ml of Tri-Sil/BSA. The resultant solution was chromato- graphed on a 10-ft column of Dexsil 300 on Chromosorb W/AW, DMCS- treated. The temperature was programmed at 6ø/rain between 300 ø and 350øC. The quantitative usefulness of this method has not been investigated. Triethylrhodamine The triethylrhodamine standard assayed against the material chromato- graphed on the silica gel column was 83.2 --- 4.4% pure. Three lots of D and C Red No. 37 were found to contain 2.1 ñ 0.1%, 1.4 ñ 0.0%, and 1.9 + 0.5% triethylrhodamine. Triethylrhodamine is, in the solid state, an unstable material. After purifica- tion by chromatography it gives one component by two-dimensional chroma- tography. After drying in vacuo or precipitation from ether as the hydro- chloride, it reverts to a mixture almost identical to the material before purifi- cation. The experiment demonstrating this is given below. Fifty milligrams of unpurified tr,•ethylrhodamine was placed on a 2-mm preparative silica plate in a band 18 em long. It was migrated with a solvent containing $0 parts of methanol: 70 parts of CHC13: I part of acetic acid. The triethylrhodam'ne band was scraped off and eluted from the silica with methanol. The solution was then concentrated to 15 ml in a stream of nitrogen and placed on another preparative silica plate. This t'me it was migrated with a solvent containing 20 parts of dimethylformam!de and 20 parts of diehloro- methane. As before, the triethylrhodamine band was scraped off and eluted. The eluate was concentrated as above and poured into 400 ml of ether con- taining 1.0 ml of 1.87N HC1 in methanol. The hydrochloride was allowed to crystallize overnight at 4øC. The crystals were washed with ether and dried at room temperature in a stream of nitrogen. The unpurified material and triethylrhodamine purified as above were ehromatographed by two-dimensional chromatography using the solvents above. The results of this chromatography are shown in Fig. 3. It can be seen that 21 of-the 24 original spots are present,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)