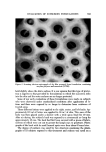
I. Soc. Cosmet. Chem., 24, 577-592 (August 19, 1973) The Synergistic Effects of Nonionic Surfactants upon Cationic Germicidal Agents IRVING R. SCHMOLKA, Ph.D.* Presented March 1, 1972, before the New York Chapter, Clifton, N.J. Synopsis-The mechanism by which cationic SURFACTANTS are believed to kill micro- organisms has been reviewed, a number of published reports on the antagonistic ef- fect of nonionic surfactants on GERMICIDAL COMPOUNDS have been examined, and the mechanism for this inactivation has been explained. Some examples of SYNERGISTIC EFFECTS have been reviewed. Despite the method by which these activities are meas- ured, the importance of explaining results in terms of micelles and the CRITICAL MICELLE CONCENTRATION (cmc) has been explained. Suggestions have been made as to how the cmc can be increased and thereby lessen the possibility of reducing the activity or even obtaining synergistic germicidal activity of a cationic surfactant in the presence of nonionic surfactants. INTRODUC•ON The presence of harmful microbes in cosmetic products is becoming a mat- ter of greater public concern. There appears to be an increased consumer awareness for products that are not only innocous from a microbial point of view when the housewife first makes her purchase, but should remain so dur- ing shelf life and usage in the home. In 1968, it was stated (1) that the problem of bacterial contamination was one of the most important which faced the cosmetic industry at that time. That there is a need to provide better control over microorganisms in the cosmetic industry was underscored by the disclosure of Wolven and Leven- stein (2) that about % of approximately 200 cosmetic products available in the U.S., which they had examined, were contaminated with harmful micro- organisms. Rather than considering the many factors that can be involved in *BASF Wyandotte Corp., Wyandotte, Mich. 48192. 577
578 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS the contamination of cosmetic products, an attempt will be made to establish guidelines for developing a microbiologically acceptable nonionic-based cosmetic product containing a germicidal cationic surfactant. This informa- tion is especially important today with all the product changes going on, in order to meet government regulations. In 1971, Saad and Shay (3) pointed out the necessity for utilizing physical chemistry in the cosmetic industry. This paper might be considered a mar- riage of the physical chemist and the microbiologist, with a microbiologically acceptable cosmetic product as the offspring! More specifically, the cause of the interaction between a cationic quaternary surfactant and a nonionic sur- factant will be explained, so that when both are used in a product, it may be possible for the formulatot to at least maintain, or possibly enhance, the ger- micidal activity of the cationic surfactant, rather than inhibit it. After the theoretical portion is concluded, some practical suggestions are offered to en- able the cosmetic formulator to cope with this rather complex problem. First, some of the terms which will be used, will be defined, so as to elimi- nate any possible misunderstanding. The nonionic surfactants will be limited to those surface-active agents which are derived from the addition of ethylene oxide to a water-insoluble hydrophobe, such as a fatty alcohol, a fatty acid, an alkylphenol, a sorbitan fatty ester, or a polyoxypropylene glycol, etc. Exclud- ed from this consideration are such molecules as the fatty alkanolamides or amine oxides. Second, for the cationic germicidal compound, reference is made to N- alkyldimethylbenzylammonium chloride, or cetyl pyridinium chloride, and other structurally similar compounds which contain a positively charged ni- trogen atom with four substituents. These are commercially available as BTC©, • Hyamine 3500'9,? Tetrosan© 3,4D,* Beloran©,* Emcol E-607©,õ etc. All are characterized by their ability to kill microorganisms. Third, only those systems in which water is the dominant or exclusive sol- vent or continuous phase will be considered. Therefore, cosmetic products which are emulsions of water in oil will be excluded. Synergism has been defined (4) as the use of two or more different agents which results in greater killing. It is defined here as the ability of a nonionic surfaetant, which is itself devoid of any antimicrobial activity, to increase the germicidal property of the cationic surfactant over that of the cationic surfac- rant alone. If the nonionic surfactant itself should exhibit some antimicrobial property, then the synergistic effect would be defined as the ability of a mix- ture of cationic and nonionic surfactants to exhibit greater germicidal activity than the sum of the germ-killing properties ot• the two individual surfactants. Onyx Chemical Co., lersey City, N.I. Rohm & Haas Co., Philadelphia, Pa. Ciba-Geigy Corp., Ardsley, N.J. Witco Chemical Corp., New York, N.Y.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)