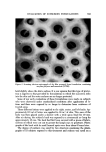
EVALUATION OF SUNSCREEN FORMULATIONS 549 Table V Arm Spray-Ultraviolet Analysis of Sunblock Products Absorption Product Before After % Loss Formulation 2 0.75 0.41 45 Commercial Sunblock A 0.62 0.06 90 Commercial Sunblock B 0.79 0.05 94 Field testing of our sunblock, Formulation 2, was conducted at Concord, Calif., under conditions of intense ultraviolet exposure. Three challenge con- ditions were used: long-term sun exposure with no external insult, water in- sult with sun exposure, and perspiration insult with sun exposure. Commercial Sunblocks A and B were used for comparison in all three test conditions. A total of 61 male and female subjects participated in this test. Two products •vere applied to each subject in a half-body type test. After challenge the sub- iects were exposed to 2-4 hours of midday sun and color evaluation was made after 5 hours by three observers. Photographs of each subject were also taken. The color development was rated on the following scale: 0, no color de- velopment 1, slight color 2, medium color 3, intense color. Table VI shows that with 4-hour sun exposure there has been more color development in the case of Sunblock A than with Formulation 2. Table VI Sun Exposure (4 Hours)-No Insult Product No. Subjects Ave. Color Formulation 2 8 1.3 Commercial Sunblock A 8 2.0 Formulation 2 8 1.6 Commercial Sunblock B 8 1.8 An exposure to 45 min of swimming and 21/2 hours of sun resulted in marked differences of color development between Formulation 2 and the commercial sunblocks (Table VII). The effects of a 20-min sauna with its resultant per- spiration challenge is shown in Table VIII. Again, the improvement due to Formulation 2 is demonstrated although the challenge does not seem to be as severe as the swimming tests. Table VII Water Insult (a/• Hour)-Sun Exposure (21/2 Hours) Product No. Subjects Ave. Color Formulation 2 9 1.3 Commercial Sunblock A 9 2.5 Formulation 2 20 1.2 Commercial Sunblock B 20 2.6
55O JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table VIII Perspiration Insult (% Hour)-Sun Exposure (3 Hours) Product No. Subjects Ave. Color Formulation 2 8 1.0 Commercial Sunblock A 8 1.8 Formulation 2 8 1.2 Commercial Sunblock B 8 1.9 SUM.1VIAR¾ The scanning electron microscope showed a unique sponge-like structure of polymeric films containing sunscreens. This structure explained the im- proved sunscreen retention on the skin as well as the reduction of oily feel of these materials. The entire system had to be in proper balance with appropri- ate ingredients and a unique polymer. Several evaluation techniques were used to determine the e$cacy of those polymer formulations. These included rather standard spot-burning methods, spectrophotometric analysis of alcohol extracts of the skin, fluorescent tracer techniques showing distribution and retention of sunscreen, standard photo- graphic methods, and visual inspection. The sunscreen retention was followed by means of the above tests in labora- tory water spray procedures, 45-min swims, and sauna baths with sun expo- sure. Polymer formulations showed superior sunscreen retention over commer- cial products in both laboratory and field tests. Certain aesthetic advantages also were noted such as nongreasiness, ease of application, and no sand pick- Up. (Received December 12, 1972) REFERENCES (1) Kligman, A.M., Early destructive effect of sunlight on human skin, J. Amer. Med. Ass., 9.10, 2377 (1909). (2) Pathak, M. A., Fitzpatrick, T. B., and Frenk, E., Evaluation of topical agents that prevent sunburn-superiority of p-aminobenzoic acid and its ester in ethyl alcohol, N. Engl. J. Med., 9,80, 1459-03 •1909).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)