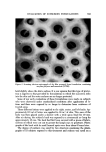
DETERMINATION OF TRACE COMPONENTS IN DYE 559 0 O o (b l! after PLC o 0o o Figure 3. Two-dimensional thin-layer chromatography of triethylrhodamine
560 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Similarly, when a solution of chromatographically purified triethylrhoda- mine is rechromatographed by tic prior to evaporation to dryness as the base, a single spot is obtained. After evaporation to dryness, the material rapidly breaks down into a multicomponent system. SUMMARY Phthalic acid and m-diethylaminophenol in D and C Red No. 37 have been assayed by the gas-liquid chromatography of their trimethylsilyl derivatives. o-(2-Hydroxy-4-diethylaminobenzoyl) benzoic acid in the same dye has been assayed by both thin-layer and gas-liquid chromatography of the trimethyl- silyl derivative. Triethylrhodamine has been assayed by tlc but the assay is complicated by its apparent instability. ACKNOWLEDGMENT The authors wish to express their thanks to Mr. Richard Schnetzinger who synthesized the triethylrhodamine and the HDBA. (Received October 26, 1972 ) REFERENCES (1) Graichen, C., Report on intermediates derived from phthalic acid, J. Ass. O•c. Anal. Chem., 33, 398 (1950). (2) Graichen, C., Report on intermediates derived from phthalic acid. Phthalic acid in D and C Yellow No. 10, Ibid., 34, 407 (1951). (3) Harrow, L. S., Determination of m-diethylaminophenol in D and C Red No. 19 and D and C Red No. 37, Ibid., 34, 133 (1951). (4) Ritchie, C. D., Wenninger, J. A., and Jones, J. H., Studies on coal-tar colors. XIII. D and C Red No. 19: Identification and determination of triethylrhodamine and o-(2-hydroxy-4-diethylaminobenzoyl) benzoic acid in commercial samples of D and C Red No. 19, Ibid., 42, 720 (1959).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)