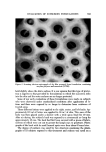
SYNERGISTIC EFFECTS OF NONIONICS ON CATIONICS 589 There are several published reports that the block polymer nonionics form micelies. Cowie .and Sirianni (24) have reported that only 2-8 molecules are needed to form a micelle. However, the published data indicate that the micelles of the block polymers are unlike the micelles of other nonionic sur- factants. The cmc values of the block polymers have been reported (25) to increase with increasing temperature and in the presence of added electro- lytes. This is the opposite of what has been reported for alkylphenol ethoxy- lares (26-28). Additional interesting information was obtained from a doctoral dissertation of Sheth (29), in which he studied solubilization using surfac- rants. He compared two block polymers, Pluronic F68 and Tetronic 908, with Bri i 35©* and Triton WR-1339. Sheth found a difference in solubilization be- tween the two groups, and concluded that with the block polymers, solnbili- zation occurs in the outer layer of the micelle, while with the latter, solubili- zation occurred within the interior of the micelle as well. CONCLUSION It now appears quite obvious that miceliar formation plays a significant role in the interaction of nonionic surfactants with quaternaries insofar as their effect on germicidal activity is concerned. How, then, should the cosmetic chemist go about formulating new products which will have no antagonistic germicidal effects and even possibly create a synergistic germicidal effect? The following steps are suggested: 1. A low ratio, 4/1 or less, of nonionic surfactant to cationic should be used. As the data (14) have indicated, better germicidal activity is obtained under these conditions than if a high ratio of these two types of surfactants is used. 2. Nonionic surfactants with higher critical micelle concentration values should be used. This means that there are fewer micelles and that there will be less chance of the quaternary being inactivated. As one increases the num- ber of moles of ethylene oxide on a given hydrophobe, the cmc value often increases. Thns, Hsiao et al. have reported (27) this occurs with nonylphenol ethoxylates on which the moles of ethylene oxide increase from 10.5 to 30. In Table IV, data were shown that as the length of the ethylene oxide chain on the alkylphenol increases, the killing time decreases. As one possibfiity, non- ionic surfactants should be selected with as large an ethylene oxide .chain as possible, or nonionic surfactants with a higher HLB ratio should be used, in order to at least maintain the original germicidal activity of the quaternary. In studying micelle formation in mixtures of nonionic and cationic surfac- tants, Schick investigated (30) a series of dodecyl alcohol ethoxylates in the presence of a quaternary. He reported that the shielding capacity of the oxy- ethylene chain becomes more pronounced with increased length of the oxy- ethylene chain. *Arias Chemicals Div., ICI America Inc., Wilmington, Del.
590 JOUBNAL OF THE SOCIETY OF COSMETIC CHEMISTS 3. The effect of adding urea to the system should be considered. Thus, it has been reported (26) that the addition of urea to a nonionic surfaetant in- creases the hydration in the hydrophile and increases the critical mieelle con- centration of the surface-active agent. Since urea is already found on and in humans, there should be little or no difficulty in getting clearance from the EPA or FDA to use this synthetic organic compound in new cosmetic prod- ucts. And since urea is an inexpensive chemical, this approach should be of great interest. 4. The effect of using block polymer surfaetants should be considered rather than those with a lipophilie hydrophobe. The fact that the mieelles of the block polymers are different may, in some areas of application, enable the cosmetic chemist to obtain a superior product, as has already been pointed out for the detergent sanitizer. These same data may be useful in substantiating the efiieaey of products which wish to make the claim, "kills gertns on con- tact."The fact that many of the block polymers have good toxicity data available should serve to stimulate their consideration. 5. The use of an anionie surfaetant should be considered together with the nonionic m•d eationic surfaetants. Thus, Moore and Hardwick have reported (16) that they found the addition of an anionie to a mixture of a quaternary ammonium compound and a nonionic surface-active agent gave germicidal activity considerably higher than expected. They attributed this to the anionie acting as an inaetivator towards the nonionic, although in a three component system of mixed mieelles it would be expected that the anionie would more strongly inaetivate the eationic than the nonionic. However, the reduction in baeteriostatie effect may be far less than reduction in bactericidal activity. Thus Bouehal (31), using a mixture of an anionie, eationic, and nonionic sur- factant in his products, reported flint the antibacterial potency of a quater- nary compound was increased noticeably by addition of certain anionie sur- factants, specifically sodium N-lauroyl sareosinate. The addition to this mix- ture, of certain water soluble nonionic surface-active agents containing a polyoxyethylene chain of 20 to 700 carbon atoms ( 10 to 350 moles of ethylene oxide), increased the antibacterial power further. This latter group of non- ionic surfaetants included fi•e Pluronie polyols and Renex©* 30. 6. The effect of adding other solvents, such as alcohols, glyeols, etc., which will alter the critical mieelle concentration, should be also considered. 7. The effect of changing the pkI should be tried. Cationic surfaetants gen- erally exhibit a maximum activity at a specific pkI, 7-8, and nonionic sur- factants tend to act as eationic surfaetants in acid media, due to fortnation of oxonium ions. The effect of varying the pkI on the germicidal activity should be studied to see whether or not any enhancement can be obtained in this fashion. *Atlas Chemicals Div., ICI America Inc., Wilmington, Del.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)