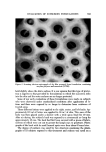
SYNERGISTIC EFFECTS OF NONIONICS ON CATIONICS 583 surfactants. Thus, Gershenfeld and Stedman found (10) that activity of cetyl- pyridinium chloride and cetyltrimthylammonium bromide was enhanced at low nonionic surfactant concentrations but inhibited at higher surfactant con- centrations. The antagonistic effects of an oxyethylated tall.ow alcohol on the bacteriostatic properties of cetyltrimethylammonium bromide were reported (11) by Davis, while Ritter reported (12) that Tween 80 neutralized the bactericidal effect of cetylpyridinium chloride. Nine different quaternary am- monium compounds were studied by Barr and Tice (13) in the presence of 5% Tween 60. The data, as shown in Table II, indicate that only benzalkonium chloride was effective at a concentration of 0.1%. Wedderburn studied (14) the preservation of toilet preparations containing various nonionic surface agents at 2% concentration, and several cationic germicides, including benzaL konium chloride at 0.1% concentration, and obtained either partial or com- plete inactivation in all cases. The effect of varying the ratio of Tween 80 to benzalkonium chloride was then studied using four different test organisms, two gram-positives and two gram-negatives. The data in Table IH are based on the use of nutrient broth or agar as the culture medium. The test media were inoculated with dilutions of each culture to determine the great- est number of bacteria against which each concentration of preservative was effective. Other reports of inactivation of quaternary ammonium compounds by non- ionics have been given by Beckett and Robinson (15) and by Moore and Hardwick (16). Their studies indicated that in very dilute solutions of the non- ionic surfactant there was an increase in the effectiveness of the germicidal activity of the quaternary ammonium compound, but at higher nonionic con- centrations the activity of the cationic was greatly reduced. Moore and Hard- wick explained this on the basis of mixed micelle formation occurring, with a gradual increase in activity occurring, towards gram-negative organisms, at Table II Effectiveness of Various Quats as Preservatives for 5% w/v Solutions of Tween 60 • P. M. A. Quat % w/v Aeruginosa • Albicans Niger Contro! Cetylpyridinium C1 0.10 + + + CTAB 0.10 Benzalkonium C1 0.10 -- -- -- Hyamine 3104 0.10 + + + Hyamine 2389 0.10 + q- + Hyamine 1622 0.10 + + Hyamine 10-X 0.10 q- q- q- G-271 1 q-S q-S q-S G-251 1 q-S q-S q-S None ... q-S q-S q-S •From Barr and Tice (13), reproduced with permission of copyright owner. bq-: Growth observed, ----• No growth observed, S: Splitting of ester observed.
584 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table III Effect of Ratio of Nonionic to Quat ß Organism * 0.1% 0.1% 0.1% 0.1% 0.1% 0.1% 0.1% 0.1% BAC BAC BAC BAC BAC BAC BAC BAC Alone ø q- q- q- q- q- q- q- 0.1% 0.2% 0.$% 0.75% 1.0% 1.5% 2.0% Tw. 80 Tw. 80 Tw. 80 Tw. 80 Tw. 80 Tw. 80 Tw. 80 Staph. Albus a ........ Strep. Boris a ........ B. Coli a .... q- q- q- q- b ..... + + + Ps. Fluorescens' a .... b .... + + + + •From Wedderburn (14), reproduced with permission of copyright owner. ba = 0.1 ml of a 24-hour broth culture, b = a 1/30 dilution of a. cq- _--Growth after 24 hours (preservative inactive), -- ----No growth after 72 hours (pre- servative active). first, followed by a gradual decrease in activity due to depletion of the con- centration of the single ion of the quaternary ammonium compound, by incor- poration into the micelle of the nonionic, thus reducing the effective germi- cidal concentration. With gram-positive organisms the data were of little prac- tical value because the organism was so sensitive to the effect of the quarternary ammonium compound that, in the dilutions used, the concentra- tion of nonionic was too low for its effect to be observed. They reported that the ratio of nonionic to quaternary should not be greater than approximately 4:1, otherwise marked inactivation occurred. Their work was carried out with very low concentrations, namely a quaternary concentration in the order of 10 -3 and the nonionic concentration from 0.001 to 0.5%. The names of neither the nonionic compound nor the quaternary germicidal compound were dis- closed. There have been several reports in the literature that by the addition of nonantiseptic nonionic surfactants to solutions of cationic surfactants, the anti- septic activity of the latter is always greatly increased, provided that the con- centration of the cationic surfactant approaches extreme effective dilution for germicidal activity, which was 10 -4 g/1. Delmotte and Geeraerts (17) re- ported this occurred with a nonionic concentration of 10 -2 g/1. Nonionic sur- factants which were reported to enhance the cationic activity included nonyl- phenol ethoxylates containing 8, 9, 11, 13, and 15 moles of ethylene oxide, tridecyl alcohol with 9 moles of ethylene oxide, and a 5-mole ethylene oxide adduct of oleyl alcohol. The cationic surfactants included alkyldimethyl- benzylammonium chloride, cetyltrimethylammonium bromide, and p-tolyl- dodecyltrimethylammonium methosulfate. In a subsequent report, Delmotte (18) verified these data with the nonylphenol ethoxylates, and found enhanced
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)