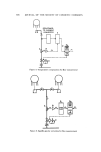
SYNERGISTIC EFFECTS OF NONIONICS ON CATIONICS 585 bacteriostatic activity at a cationic surfactant concentration of 103 to 10 4 g/1. In a continuation of this work, Delmotte expanded his investigation (19) to include 17 other nonionics, including ethoxylated sorbitan fatty esters such as Tween 20, 40, 60, and 80, ethoxylated fatty alcohols, ethoxylated fatty acid glycerides, a hexaethylene glycol monostearate, such as Be'•sa•n IIi©*, and a polyethylene oxide-polypropylene oxide block polymer, Pluron'_c F68. Gen- erally, most nonionics gave an increase in bactericidal activity at 10 -2 to 10 -a g/1., but a disappearance in activity at higher or lower nonionic concentra- tions. Three of the nonionics, Belsam III, Tween 40, and Pluronic F68, gen- erally caused a slight decrease in bactericidal actkvity while some others gave irregular results. Delmotte and coworker, in all their experiments, scrupulously adhered to the Bondi (20) method, and measured differences in diameters of zones of inhibition. Is this a valid measure of synergistic act:vity? Gucklhorn (21) looked with disfavor upon data obta'ned from measurements of zones of inhi- bition. He believed this method has a serious disadvantage because it mea- sures the rate of diffusion of the preservative through an agar medium, which is not at all necessarily connected with antimicrobial power. This point should be carefully considered. It is suggested that the data which are obtained by this technique represent the sum of (a) the actual kill- ing power of the unbound cationic and (b) the wetting or spreading power of the mixed surfactant system, and are illustrated in Fig. 2. Example I indicates antagonism, Example II represents no change, and Example III depicts synergism. The last shows that the total germicidal ac- tivity can be due to (a) a large increase in wetting combined with a decrease in germicidal activity due to mixed micelie formation, (b) no change in ger- micidal activity plus a moderate increase in wetting, or (c) a true synergistic effect of the germicidal activity plus a small increase in wetting. The only way actually to measure the true synergistic enhancement of the germicidal activity of the cationic surfactant would then be to repeat the evaluation of the specific nonionic and cationic system by the critical kill test, or use dilu- tion method over a range of organisms, including P. aeruginosa, S. aureus, or by some type of bacteriostatic method of evaluation, since cosmetic chemists are more concerned with preservation or bacteriostasis of their products. Un- fortunately, the corresponding data for these nonionics by another method were not obtained. Another way would be to determine whether or not a lower cationic concentration in the presence of a fixed nonionic concentration would give the same zone of inhibition as a higher cationic concentration gives, in the absence of the nonionic surfactant. That the wetting property of a nonionic may be important, in this particu- lar test method, was substantiated from other data obtained by De]motte *Union Chimique Belge, Bruxelles, Belgium.
586 JOURNAL OF THE SOCIETY OF COSMETIC CItEMISTS ANTAGONISM NONIONIC .I, Q• :r II • NO CHANG III $¾NERGISN EXAMPI'E III 6ram , ..% 12rn rn DIAMETER DIAMETER a 4 mm QUAT 4- 8ram W.A. b 6 m m QUAT-I- 6mm W.A. c 8 mm QUAT -!- 4ram W.A. Figure 2. Zones of inhibition (oeg), in which he evaluated Pluronic polyol P103, one of the block polymers with better wetting properties and found a significant enhancement of germicidal activity under certain concentration conditions. It is suggested that the agar disk technique is meaningful when one thinks of synergism. If one adds a nonionic surfactant, which is also a good wetting agent, to a cationic surfactant in products as diverse as a mouth wash, a skin cleanser, a douche, an antiperspirant cream, and others, the product with the nonionic wetting agent will spread out over a wider surface area than the same product without the nonionic in 99% of the cases. If the product has equalled or exceeded its original germicidal activity, and this would have to be determined by an in vitro test, then one would have to accept this as a synergistic activity. The wetting action of a nonionic surfactant may not be divorced or isolated from its other physical properties or contributions to a formulated system. The end product is a composite of all properties of all ingredients. One other source of data relates to germicidal compositions containing both nonionic and cationic surfactants. In a U.S. patent on detergent sanitizers (9.3)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)