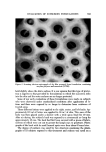
SYNERGISTIC EFFECTS OF NONIONICS ON CATIONICS 581 cationic surfactant needed to cause bacterial dissolution, by providing low- ered interfacial tension and hence a more rapid passage through the proto- plast •nembrane barrier. In general, such is not the case. In fact, there are only a few references to a synergistic effect. Generally, it is reported that there is a decrease in bacterial activity of a germicidal agent in the presence of a non- ionic surfactant. Thus, DeLuca and Kostenbauder have reported (9) that in the presence of Tween 80©*, the inhibitory concentration of two cationic germicides, cetylpyridinium chloride and benzalkonium chloride, towards Aerobacter aerogenes was reduced by a factor of 100 to 1000, depending on concentration. A similar reduction was observed with Triton X-100©* and to a lesser extent, xvith Pluronic L82© . These data are shown in Table I. These same authors studied the degree of interaction between 0.2% Tween 80 and varying concentrations of two cationic surfactants, cetylpyridinium chloride and cetyldimethylbenzyl ammonium chloride, as shown in Fig. 1. Tahl• T Influence of Some Nonionic Surfactants on Concentrations of Cationic Bequired to Inhibit Aerobacter Aerogenes a Inhibitory Concentration Cetylpyridinium Benzalkoniurn Nonionic C1 C1 0 1-100,000 to 1-250,000 No growth at 1-100,000 0.5% Tween 80 1-2500 to 1-500 ... 2.0% Tween 80 1-250 to 1-500 3.0% Tween 80 • 1-100 to 1-250 1-500' •& 1-1000 3.0% Triton X100 • 1-100 to 1-250 ... 3.0% Pluronic L62 • 1-500 to 1-1000 ... "From DeLuca and Kostenbauder (9), reproduced with permission of copyright owner. •Treated with ion exchange resin before use. These data have been interpreted in terms of miceliar formation. Not only does the nonionic surfactant form micelies, but the cationic surfactant also possesses this property. A micelie is an aggregation of molecules or the forma- tion of a new phase, which does not separate out of solution. The minimum concentration at which this occurs is known as the cmc, or critical micelle concentration. This property aggregation, or micelie formation, is reflected in marked changes in solution properties, including surface tension, refractive index, solubility, electrical conductivity, etc. In the presence of both types of surface-active agents, mixed •nicelles form. The data in Fig. 1 can be inter- preted as due to solubilization of part of the cationic surfactant in the micelles Atlas Chemicals Div., ICI America Inc., Wilmington, Del. Rohm & Haas Co., Philadelphia, Pa. $BASF Wyandotte Corp., Wyandotte, Mich.
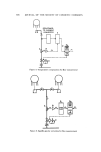
582 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 4O • 30 • 20 m 10 A 10 30 50 Free Quat (M/L x 104 ) Figure I. Adsorption isotherms for binding of quats in 0.9.% Tween 80 [from (9), produced with permission of copyright owner] A. Cetylpyridinium chloride B. Benzalkonium chloride re- ooe the Tween 80. Thus, the quaternary ions are present at a lower concentra- t-ion than is found with the same use concentration in the absence of the non- ionic. Figure 1 illustrates the binding or complexing ooe the two cations, both above and below their critical micelie concentration. At the lower cationic concentration, the quaternary is absorbed within the nonionic micelie. As the concentration of cationic increases, it continues to form micelies and the activ- ity of the cation passes through a maximum in the vicinity of the critical mi- celie concentration, which is the peak on the curve. Once past the cmc, the additional cationic surfactant is no longer complexed, and acts as the free surfactant. Several other investigators had reported their observations on the activity of eationic surfactants in the presence of varying concentrations of nonionic
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)