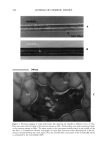
450 JOURNAL OF COSMETIC SCIENCE Both forms interconvert easily by oxidation-reduction reactions (1) (Figure 1). In recent years, the beneficial role of the ot-lipoic acid/dihydrolipoic acid redox couple has cap- tured the interest of many authors since it has been referred to as a "universal antioxi- dant" that functions in both membrane and aqueous phases (4-6). The metabolic role of ot-lipoic acid (1) and its reduced form, dihydrolipoic acid (2), has been known for decades, whereas only recently a great deal of attention has been given to it as a possible antioxidant. Chatgillialoglu and co-workers (7) have studied thiyl radical catalysis and the influence of antioxidant vitamins in the geometrical isomerism of monosaturated fatty acids. The thiyl radical catalyzed cis-trans isomerization of monounsaturated fatty acids indepen- dent of the position and length of the chain. The behavior of 1 and 2 in the cis-trans isomerization reaction is similar to that described for thiyl radicals generated either from thioIs or disulfides. The inibition of the isomerization process due to antioxidants increased in this order: ot-tocopherol ascorbic acid all-trans retinol. Lipoic acid dissolves in lipids (8). This favors its rapid penetration in all the portions of the cell, where it confers protection to the lipidic cell membrane, to the aqueous compartments, and to the cell nucleus. Its physical and chemical properties are funda- mental for cellular metabolism: it increases the levels of antioxidant vitamins C and E, grants protection against the damage caused by free radicals, and acts synergistically with other antioxidants. The aging process is considered to be similar to that produced by inflammation because both are mediated and perpetuated by the activity of free radicals. Therefore, anything causing inflammation in the cells accelerates the aging process, while prevention has the opposite effect (9-11). For this reason, the use of a topical form of lipoic acid is proposed both for the treatment of cutaneous aging and for the prevention of the erythema associated with exposure to A SH SH B Figure 1. (A) ot-lipoic acid. (B) ot-dihydrolipoic acid (reduced form).
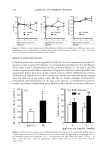
LIPOIC ACID STABILITY 451 ultraviolet radiation (12). The purpose of this work is to study the stability of lipoic acid in the presence of vitamins A and E in o/w emulsions for cosmetic application. MATERIALS AND METHODS MATERIALS Vitamin A (as palmitate) and vitamin E (as acetate) were provided by Merck (Germany) and lipoic acid was provided by Labochim (Laboratorio Chimico Internazionale, Italy). The emulsions consisted of silicone fluid (Dow Corning, Brazil) mineral oil, and pe- troleum jelly (R.A.A.M., Argentina) and acetylated lanolin, Acelan L (Fabriqu•mica, Argentina) as oil phase polyoxyethylenated fatty alcohol, Ceral PW (Fabriqu•mica, Argentina) as surfactant methyl p-hydroxybenzoate and propyl p-hydroxybenzoate (Clariant, United Kingdom) as preservatives and propylene glycol (Dow Chemical, USA) and demineralized water as hydrophilic phase. Oil-in-water-type emulsions con- taining lipoic acid (system 1) lipoic acid and vitamin A (system 2) lipoic acid and vitamin E (system 3) lipoic acid and vitamins A and E (system 4) and a drug-free emulsion (system 5) (Table I) were prepared. PREPARATION OF THE EMULSIONS 1. The non-ionic emulsifier was melted into a stainless steel container then acetylated lanolin, silicone fluid, propyl p-hydroxybenzoate, and mineral oil were added. It was mixed by slow agitation, avoiding the incorporation of air and keeping the tempera- ture between 72øC and 74øC. Lipoic acid was then added. It was stirred, and the temperature was maintained until a full dispersion was obtained. 2. Demineralized water, propylene glycol and methyl p-hydroxybenzoate were mixed into another stainless steel container. This mixture was heated to 75øC. 3. Both phases were filtered by gravity filtration. Then mixture 1 was incorporated into mixture 2, and stirred at 900 rpm for five minutes. Cooling was then started and stirring was slowed down. Table I Composition of Emulsions Systems Materials (g/100 g) 1 2 3 4 5 Polyoxyethylenated fatty alcohols 7.000 7.000 7.000 7.000 7.000 Silicone fluid 0.500 0.500 0.500 0.500 0.500 Mineral oil 5.000 5.000 5.000 5.000 5.000 Acetylated lanolin 1.000 1.000 1.000 1.000 1.000 Methyl p-hydroxybenzoate 0.200 0.200 0.200 0.200 0.200 Propyl p-hydroxybenzoate 0.100 0.100 0.100 0.100 0.100 Vitamin A palmitate 0.120 -- 0.120 -- Vitamin E acetate -- 0.400 0.400 -- Lipoic acid 0.500 0.500 0.500 0.500 -- Propylene glycol 9.000 9.000 9.000 9.000 9.000 Demineralized water 100.000 100.000 100.000 100.000 100.000
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)