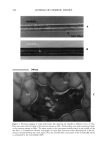
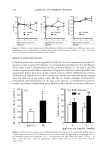
j. Cosmet. Sci., 55,463-471 (September/October 2004) Comparative study between the viscoelastic behaviors of different lipid nanoparticle formulations E. B. SOUTO, S. A. WISSING, C. M. BARBOSA, and .. R. H. MULLER, Department of Pharmacez/tics, Biopharmacez/tics and Biotechnology, Free University of Bet/in, Ke/chstrasse 31, D-12169 Bet/in, Germany (E.B.S., S.A.W., R.H.M.), and FacMty of Pharmacy, Department of Pharmacez/tica/ Technology, Oporto University, Rz/a An/ba/ Cz/nha 164, P-4050-047 Porto, Portz/ga/ (E.B.S., C.M.B.). Accepted for pz/blication JMy 7, 2004. Synopsis Application of drug substances to the skin for systemic absorption or action in a particular layer of the skin is a rather old approach. However, over the last years it has received much more attention, as a consequence of the development of new membrane-moderated and matrix reservoir devices. As new reservoir systems, solid lipid nanoparticles (SLN TM) and nanostructured lipid carriers (NLC TM) have been successfully tested for derreal application of different physicochemical substances. The knowledge obtained from theological investigations of these systems may be highly useful for the characterization of the newly developed topical formulation. In the present study, an oscillation frequency sweep test was used for the evaluation of storage modulus (G'), loss modulus (G"), and complex viscosity ('q*) of twelve different SLN and NLC formulations, over a frequency range from 0 to 10 Hz. The lipidic aqueous dispersions were prepared using three different solid lipids (Softisan©138, Compritol©888, and stearyl alcohol) as matrix material. Miglyol©812, tocoph- erol, sunflower oil, and long-chain triacylglycerols were the chosen liquid lipids for NLC preparation. The objective of the present work was to investigate the effect of these different liquid lipids on the rheological properties of aqueous dispersions of NLC as model systems. It was found that the liquid oil component of the formulation has a strong influence on the viscoelastic parameters, which are dependent on the particle size, zeta potential, and crystallinity of the lipid particles, as well as on the solid lipid used. INTRODUCTION The skin is the largest organ of the body, considered as a natural protective barrier against either the penetration of dangerous exogenous compounds or the loss of excessive amounts of water and other essential compounds from the body. At the same time, it can also be a promising portal of entry of active substances to the systemic circulation. A drug penetrates the stratum corneum and eventually it diffuses across the viable under- lying tissues, according to its physicochemical properties (1). Address all correspondence to R. H. Miiller. 463
464 JOURNAL OF COSMETIC SCIENCE A wide spectrum of formulations is available for use in topical therapy, including simple solutions, fluid emulsions and suspensions, sprays and aerosols, gels, and creams and ointments (2). These most common topical formulations are essentially able to control the release of a drug substance but not its penetration rate or residence time in the different layers. The development of the new membrane-moderated and matrix reservoir devices improve the penetration of some drugs, by targeting either to the stratum corneum or to the follicles, and control their release profiles. The use of particulate carriers for this purpose presents an interesting challenge concerning the targeting of topically applied drugs to the different skin layers and appendages. Solid lipid nanoparticles (SLN TM) and nanostructured lipid carriers (NLC TM) are novel particulate lipidic carriers that have been extensively studied for different administration routes, such as the gastrointestinal, ocular, and topical routes (3-5). Regarding topical administration, these systems show adhesive properties and, therefore, an occlusive effect, which is dependent on the size, crystalline status, and lipid composition of the particles (6,7). SLN and NLC can also protect chemically labile active ingredients in water-containing formulations against chemical degradation (4). The aim of the present study was to characterize the rheological behavior of different SLN and NLC aqueous dispersions in order to elucidate the viscoelastic improving effects of these new drug vehicles for skin application. MATERIALS Softisan © 138 was purchased from Condea (Witten, Germany), Miglyol©812 from Caelo (Hilden, Germany), and Compritol©888 and Tego Care©450 from Gattefoss• (Weil a.R., Germany). Lutrol©F68 was a gift from BASF AG (Ludwigshafen, Germany), and sunflower oil and deoxycholic acid sodium salt were obtained from Fluka Chemie AG (Steinhelm, Switzerland). Long-chain triacylglycerols (LCT) were purchased from Braun (Melsungen, Germany) and tocopherol from Sigma Aldrich (Deisenhofen, Germany). All samples developed for this study were prepared using ultra-pure Millipore water (Schwalbach, Germany) of specific resistance greater than 18 Mll-cm 1 METHODS PRODUCTION OF LIPID NANOPARTICLES According to Miiller eta/. (4), SLN and NLC aqueous dispersions were prepared by the hot high-pressure homogenization technique using the high-pressure homogenizer APV Micron Lab 40 (APV Systems, Liibeck, Germany). Briefly, the lipid components were admixed and melted at 90øC, and this liquid lipid solution was dispersed in an aqueous surfactant solution heated to the same temperature, using an Ultra-Turrax T25 (Jankle & Kunkel GmbH and Co KG, Staufen, Germany) at 8000 rpm for one minute. The obtained pre-emulsion was then homogenized at 90øC, applying three homogenization cycles at 500 bar. The produced O/W nanoemulsion was cooled down, the recrystallized lipid providing SLN or NLC aqueous dispersions.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)