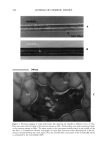
VISCOELASTICITY OF SLN AND NLC FORMULATIONS 471 logical properties of a dermatological formulation is extremely important. These studies allow (i) the evaluation of a the capability of a vehicle to suspend solids or immiscible liquids (ii) the assessment of a topical formulation with respect to patient usage, e.g., the ease of removing the preparation from a container or spreadability and adherence to the skin and (iii) the monitoring of the effect of the vehicle's consistency on the release of a drug from the preparation and its subsequent percutaneous absorption (bioavail- ability of the drug substance). The determination of G', G", and qq* as a function of the applied frequency, using an oscillation frequency sweep test, gives important information concerning topical admin- istration. According to the obtained results, for Compritol©888 as a solid lipid for NLC preparation, sunflower oil seems to be the oil that presents the best attributes for a topical formulation, i.e., more elastic behavior and a higher viscosity. With regard to stearyl alcohol, tocopherol shows the best results. The presence of a more compact and organized system can have a significant beneficial effect on the stability and on the viscoelactic properties of particulate aqueous dispersions and, therefore, on the topical administration of drug substances. REFERENCES (1) B. W. Barry and B. Warburton, Some theological aspects of cosmetics, J. Soc. Co, met. Chem., 19, 725- 744 (1968). (2) J. Ceulemans, L. van Santvliet, and A. Ludwig, Evaluation of continuous shear and creep rheometry in the physical characterisation of ointments, Int. J. Pharm., 176, 187-202 (1999). (3) R.H. Miiller, W. Mehnert, J.-S. Lucks, C. Schwarz, A. zur Miihlen, H. Weyhers, C. Freitas, and D. Rtihl. Solid lipid nanoparticles (SLN)--An alternative colloidal carrier system for controlled drug delivery, Eur. J. Pharm. Biopharm., 41, 62-69 (1995). (4) R. H. M•iller, K. M•ider, and S. Gohla, Solid lipid nanoparticles (SLN) for controlled drug delivery-- A review of the state of art, Eur. J. Pharm. Biopharm., 50, 161-177 (2000). (5) W. Mehnert and K. M•ider, Solid lipid nanoparticles--Production, characterization and applications, Adv. Drug Deliv. Rev., 47, 165-196 (2001). (6) S. A. Wissing, A. Lippacher, and R. H. Miiller, Investigations on the occlusive properties of solid lipid nanoparticles (SLN),J. Cosmet. Sci., 52, 313-323 (2001). (7) S. A. Wissing. SLN alr innovatives Formulierungskonzept J•ir pflegende und protective dermale Zubereitungen, PhD Thesis, Free University of Berlin, 2002. (8) R. H. Miiller, "Zetapotential und Partikelladung in der Laborpraxis," in Wissenschaftliche Verlag•gesdl- schaft mbH (Stuttgart, 1996). (9) C. Freitas and R.H. Miiller, Correlation between log-term stability of solid lipid nanoparticles (SLN TM) and crystallinity of the lipid phase, Eur. J. Pharm. Biopharm., 47, 125-132 (1999). (10) A. Lippacher, R. H. Miiller, and K. M•ider, Preparation of semisolid drug carriers for topical appli- cation based on solid lipid nanoparticles, Int. J. Pharm., 214, 9-12 (2001). (11) A. Lippacher, R. H. Miiller, and K. M•ider. Semisolid SLN TM dispersions for topical application: Influence of formulation and production parameters on viscoelastic properties, Ez•r. J. Pharm. Bio- pharm., 53, 155-160 (2002). (12) E. B. Souto, S. A. Wissing, C. M. Barbosa, and R. H. Miiller, Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations, Eur. J. Pharm. Biopharm. 58, 83-9O (2OO4). A. Lippacher, R. H. Miiller, and K. M•ider, Investigations on the viscoelastic properties of lipid based colloidal drug carriers, Int. J. Pharm., 196, 227-230 (2000). A. A. Zaman, P. Singh, and B. M. Moudgil, Impact of self-assembled surfactant structures on the theology of concentrated nanoparticle dispersions, J. Colloid. Interface Sci., 251,381-387 (2002). (13) (14)
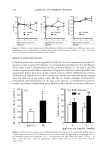
j. Cosmet. Sci., 55,473-479 (September/October 2004) Topical application of Bifidobacterium-fermented soy milk extract containing genistein and daidzein improves rheological and physiological properties of skin KOUJI MIYAZAKI, TOMOKO HANAMIZU, TOSHIRO SONE, KATSUYOSHI CHIBA, TAKASHI KINOSHITA, and SATOSHI YOSHIKAWA, Yakult Central Institute for Microbiological Research, Kunitachi, Tokyo (K.M., T.H., T.S., K.C.), and Yakult Fujisawa Cosmetics Plant, Fujisawa, Kanagawa (T. K., S. Y. ), Japan. Accepted for publit?•tion August 26, 2004. Presented in part at the 47th Annual Meeting of the Society of Cosmetic Chemists of Japan, Tokyo, September 21, 2000. Synopsis The authors examined the effects of Bifidobacterium-ferrnented soy milk extract (BE) containing genistein and daidzein on the hyaluronic acid (HA) content and rheological and physiological properties of hairless mouse and/or human skin. Topical application of BE for six weeks significantly restored changes in the elasticity and viscoelasticity of mouse skin, increased the HA content, and hydrated and thickened mouse skin. Also, topical application of a gel formula containing 10% BE to the human forearm for three months significantly lessened the decrease in skin elasticity. Therefore, BE is expected to become a new cosmetic ingredient to prevent the loss of skin elasticity through enhancement of HA production. INTRODUCTION Cutaneous aging not only induces wrinkles, dryness and thinning, but also decreases elasticity and increases viscoelasticity of the skin (1). Also, the age-dependent loss of cutaneous hyaluronic acid (HA), having specific rheological characteristics, good water- holding capacity, and certain important biological roles (2), has been hypothesized to contribute to the alterations observed in intrinsically aged skin (3,4). All-trans-retinoic acid (RA), a potent stimulus for the production of HA in vitro (2) and in vivo, as well as for thickening of the human epidermis (5), is clinically administered for not only photoaged skin (5), but also intrinsically aged skin (6). However, RA acts as an irritant on human skin, especially in Asians (7). Address all correspondence to Kouji Miyazaki. 473
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)