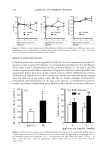
452 JOURNAL OF COSMETIC SCIENCE 4. Vitamins A and E were incorporated at 55øC. The emulsions were stored for six months at room temperature and were analyzed under the same conditions in all cases. PHYSICAL STABILITY OF THE SYSTEMS The centrifuge model was performed to study the physical stability of the systems. The centrifuge technique, based on theorical principles reflected in the Stokes formula, was used as one of the ways for predicting the vulnerability of the emulsion-to-oil coales- cence. Centrifugation was performed for 30 minutes at 3500 rpm at room temperature on a Rolco (Argentina). centrifuge. Ten-milliliter samples in graduated centrifuge tubes were used. The rating adopted was: Good: no creaming or phase separation was observed. Poor: a considerable creaming and/or phase separation was observed. pH DETERMINATIONS The pH data for all the systems were obtained with model Antares IV Parsec equipment. The pH was measured as directed in USP 26 (791), using an indicator glass electrode. The buffers solutions for standardization were from Merck (Darmstadt, Germany) at pH 4.00 and 7.01. RHEOLOGICAL DETERMINATIONS Rheometric data for all the systems were obtained by means of a Brookfield rotational viscometer (model RVT with Hellpath), employing spindle T-B at 5 rpm at room temperature. 100000 90000 80000 70000 60000 50000 40oo0 •oooo 20000 lOOOO System 1 System 2 System 3 --•- System 4 --•-- System 5 0 10 20 30 40 50 60 70 80 90 Figure 2. Viscosity of systems 1, 2, 3, 4, and 5. lOO
LIPOIC ACID STABILITY 453 Table II pH of the Systems at Initial (t - 0) and Final (t = 6 months)Time System pH (t - 0) pH (t = 6 m) 1 2.74 3.53 2 2.78 3.22 3 2.80 3.3O 4 2.80 3.46 5 5.80 5.90 Table III Selectivity: Degradation Conditions of Lipoic Acid Condition Time (h) % Recovered RRT of degradation products Water, refluxed 0.5 95.1 None detected Daylight 96 0.0 None detected Acid 1N HC1, refiuxed 0.5 98.1 0.65 Hydrogen peroxide, 100 vol., refluxed 0.5 0.0 None quantified RRT: relative retention time. Table IV Selectivity: Degradation Conditions of Vitamin A Condition Time (h) % Recovered RRT of degradation products Water, refluxed 0.5 83.9 0.07, 0.11, 0.15, 0.17 Daylight 72 0.0 0.07, 0.10, 0.18 Acid 1N HC1, refiuxed 0.5 0.0 0.06, 0.08, 0.09, 0.12 Hydrogen peroxide, 100 vol., reluxed 0.5 73.7 0.07, 0.15, 0.18 RRT: relative retention time. Table V Selectivity: Degradation Conditions of Vitamin E Condition Time (h) % Recovered RRT of degradation products Water, refiuxed 0.5 95.7 Daylight 96 99.9 Acid 1N HC1, refiuxed 0.17 84.6 Hydrogen peroxide 0.5 93.8 100 vol., refluxed 0.1l, 0.20, 0.21, 0.26, 0.41, 0.86, 1.90 0.11, 0.20, 0.27, 0.87 0.16, 0.21,0.22, 0.58, 0.78, 1.44, 2.24, 2.49, 2.67 0.12, 0.16, 0.22, 0.48, 0.87 RRT: relative retention time. ANALYSIS OF THE ACTIVE INGREDIENTS The analyses of lipoic acid and vitamins A and E were made by HPLC. Rlateriah and reagents. The working standards employed for lipoic acid and vitamins A and E were the same as those used in the preparation of the creams. Solvents were HPLC
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)