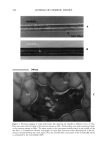
454 JOURNAL OF COSMETIC SCIENCE A B C D E Figure 3. Lipoic acid: (A) Standard. (B) Water degradation. (C) Photochemical degradation. (D) Acid degradation. (E) Oxidant degradation. A B C D E Figure 4. Vitamin E: (A) Standard. (B) Water degradation. (C) Photochemical degradation. (D) Acid degradation. (E) Oxidant degradation. grade. Water of HPLC grade was obtained by distillation and passing through a 0.45- micron membrane filter. Instrumentation. The HPLC system consisted of a dual-piston reciprocating pump (model KNK-500 G), a UV-Vis detector (model KNK-029-757), an integrator (model SP 4600) (all from Konik), and a Rheodyne injector (model 7125). HPLC conditions. The experiment was performed on an LiChrosphere © 100 RP-18 (5 pm) 125-4 column from Merck (Darmstadt, Germany) for vitamins A and E. For lipoic acid the experiment was performed on a Microsorb-MV © 100fit C18 (5pm) column (Varian Analytical Instruments, Walnut Creek, United States). The mobile phase was methanol for vitamins A and E and methanol:water (80:20, v/v), pH 3.0, adjusted with 85% phosphoric acid, for lipoic acid. Both were filtered and &gassed under reduced pressure prior to use. Separation was isocratically carried out at
LIPOIC ACID STABILITY 455 room temperature (20 ø + 2øC). The flow rate was 1.8 ml/min, with UV detection at 325 nm for vitamin A and at 230 nm for vitamin E. The flow rate was 0.6 ml/min, with UV detection at 332 nm for lipoic acid. The volume of each injection was 20 pl. In these conditions, the vitamin A, vitamin E, and lipoic acid retention times were nine, three, and six minutes, respectively. Procedure. Solutions of the vitamins and lipoic acid were prepared on a weight basis with volumetric flasks to minimize solvent evaporation. Prior to injecting the solutions, the column was stabilized for at least 30 minutes with the mobile phase flowing through the system. Quantification was accomplished using an external standard method. Each solution was prepared in duplicate and was injected in triplicate. The relative standard deviation (RSD) was below 2.0%. Working standard solutions. Twenty milligrams of vitamin A were placed into a 50-ml volumetric flask, dissolved in 40 ml of isopropyl alcohol, shaken for about five minutes, and then diluted to volume with isopropyl alcohol. The standard preparation was obtained by diluting 4 ml of the vitamin A stock solution with mobile phase to yield a concentration of 0.016 mg/ml. Fifty milligrams of vitamin E were taken in a 50-ml volumetric flask, dissolved in 40 ml of isopropyl alcohol, shaken for about five minutes, and then diluted to volume with isopropyl alcohol. The standard preparation was obtained by diluting 4 ml of the vitamin E stock solution with mobile phase to yield a concentration of 0.04 mg/ml. Twenty-five milligrams of lipoic acid were taken in a 25-ml volumetric flask, dissolved in 20 ml of methanol, shaken for about five minutes, and then diluted to volume with methanol. The standard preparation was obtained by diluting 8 ml of this acid stock solution with mobile phase to yield a concentration of 0.08 mg/ml. Preparation ofo/w samples. Around 450 mg of cream were exactly weighed, placed into a 25-ml volumetric flask, taken to volume with methanol, and shaken for about five minutes for vitamin analysis. About 450 mg of cream were exactly weighed and placed into a 25-ml volumetric flask, taken to volume with mobile phase, and shaken for about five minutes for lipoic acid analysis. The solutions were passed through a 0.45-micron membrane filter before injection. Selectivity. Method selectivity was determined by degrading vitamins A and E and lipok acid as follows: Drugs were subjected to photochemical degradation (2 ml in an open A B C D 1• Figure 5. Vitamin A: (A) Standard. (B) Water degradation. (C) Photochemical degradation. (D) Acid degradation. (E) Oxidant degradation.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)