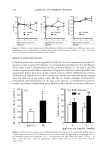
VISCOELASTICITY OF SLN AND NLC FORMULATIONS 469 [Pal U'-'-'-q •' [Pa] q* [mPas] I 250 200 ß •. 150 • 100 5O A B C Formulation 4500 4000 3500 3000 2500 2000 1500 1000 50O 0 Figure 2. Storage modulus (G'), loss modulus (G"), and complex viscosity 0q*) of the developed SLN formulations, after application of a frequency range between 0 and 10 Hz. be suitable for topical administration because they exhibit a more uniform resistance against flow. Both G' and G" moduli, as well as qq*, are also affected by the type and concentration of the surfactant in the formulation (14). Formulation B is sterically stabilized with poloxamer and, to provide a sufficient steric hindrance, a relatively large amount of this polymer is used, which might increase the effective volume fraction, resulting in higher viscosity. Therefore, the influence of the oil used for NLC preparation in its viscoelastic parameters has been evaluated in comparison to the respective SLN system. Regarding the obtained G' and G" values for NLC formulations, a completely different rheological behavior can be observed as a function of the liquid lipids of the formulations. The results are shown in Figures 3 and 4. Using Compritol©888 as solid lipid, Miglyol©812 (B L) revealed a G" value four times higher than G', and therefore this oil is responsible for a more viscous system. In contrast, when using stearyl alcohol (C1), both moduli lie in the same range of mag- nitude. Tocopherol showed a greater influence on elasticity when formulated with stearyl alcohol as a solid lipid in NLC (C2). Sunflower oil also displayed significant differences with regard to the solid lipid. In fact, when using Compritol©888 (B3) , the G' was twice as high as G", showing that the system is more elastic than viscous, but the same was not detected when using stearyl alcohol (C3). Concerning LCT, this oil did not reveal any significant difference with regard to the solid lipid used for NLC prepa- ration (8 4 and C4). Regarding the obtained qq* values, formulations B 2 and B 4 indicated a significant decrease in viscosity in comparison to the SLN system (Figure 3). Stearyl alcohol-based formulations (Figure 4) showed higher values, although the influence of the liquid oils was more pronounced in Compritol©888-based formulations (Figure 3). The lower zeta potential values of the stearyl alcohol-based nanoparticles might be responsible for the higher qq* values recorded for these systems. In fact, the decrease in electrostatic repul- sion between the suspended nanoparticles might be responsible for the develop-
470 JOURNAL OF COSMETIC SCIENCE [Pal L-7--3 G' [Pal •l* [mPas] } 250 2OO 15o 1oo 5O 0 { { • B Bi B2 B3 B4 4500 4000 3500 3000 2500 2000 1500 1000 500 0 Formulation Figure 3. Storage modulus (G'), loss modulus (G"), and complex viscosity (•*) of the developed SLN and NLC formulations based on Compritol©888, after application of a frequency range between 0 and 10 Hz. i G [Pal • G" [Pal n* [mPas] ] 350 7000 300 250 200 150 1oo 5O C CI C2 C3 6000 5000 4000 3000 2000 1000 Formulation Figure 4. Storage modulus (G'), loss modulus (G"), and complex viscosity (qq*) of the developed SLN and NLC formulations based on stearyl alcohol, after application of a frequency range between 0 and 10 Hz. ment of a three-dimensional structure more difficult to disperse due to the possible formation of aggregates. Another possible explanation is the higher mean particle size of the stearyl alcohol-based nanoparticles in comparison to the other developed formu- lations. CONCLUSIONS Although much of the qualitative information about a material can be obtained by simply observing how a sample behaves when handled, the determination of the rheo-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)