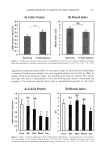
Cosmet. Sci., 55, 509-518 (November/December 2004) Effects of silicone emulsifiers on in vitro skin permeation of sunscreens from cosmetic emulsions LUCIA MONTENEGRO, DONATELLA PAOLINO, and GIOVANNI PUGLISI, Department of Pharmaceutical Sciences, University of Catania, Viale Andrea Doria 6, 1-95125 Catania, Italy. Accepted for publication October 15, 2004. Synopsis The effects of different silicone emulsifiers on the in vitro permeation through human skin of two sunscreens (octylmethoxycinnamate, OMC, and butylmethoxydibenzoylmethane, BMBM) were investigated from cos- metic emulsions. The formulations being tested were prepared using the same oil and aqueous phase ingredients and the following silicone emulsifiers: dimethicone copolyol and cyclomethicone (emulsion 1), cetyldimethicone copolyol (emulsion 2), polyglyceryl-4-isostearate and cetyldimethicone copolyol and hex- yllaurate (emulsion 3), lauryldimethicone copolyol (emulsion 4), and cyclomethicone and dimethicone copolyol (emulsion 5). The cumulative amount of OMC that permeated in vitro through human skin after 22 h from emulsions 1-5 decreased in the order 2 E 1 5 4 E 3 and was about twofold higher from emulsion 2 compared to emulsion 4. As for BMBM, no significant difference was observed in regard to its skin permeation from the emulsions being tested. In vitro release experiments of OMC and BMBM from emulsions 1-5 were performed through cellulose acetate membranes using Franz diffusion cells. Emulsions 1-3 showed an initial slow release of BMBM followed by a fast release phase, while the release of OMC showed a different pattern since the sunscreen was released very rapidly at the beginning of the experiment and then a plateau was observed followed by a second step of fast release. A pseudo-first-order release rate was observed only for BMBM from emulsion 4, while emulsion 5 released very small amounts of both sunscreens during 22 h. These findings could be attributed both to changes in sunscreen thermodynamic activity in the vehicle and to modified interactions between the active ingredient and the formulation components. The results of this study suggest that the type of silicone emulsifier used to prepare sunscreen emulsions should be carefully chosen in order to prevent the percutaneous absorption of sunscreens from these cosmetic formulations. INTRODUCTION Sunscreens are widely used in cosmetic products to protect the skin from sunburn, photoaging, and cancer (1,2). To date, UV filters have been widely tested for their ability to protect the skin from the harmful effects of UV irradiation (3), but few data have been reported on their percutaneous absorption from cosmetic formulations. An ideal sun- screen agent should remain localized close to the skin surface without penetrating into Address all correspondence to Lucia Montenegro. 5O9
510 JOURNAL OF COSMETIC SCIENCE the deeper layers of the skin. Since suntan cosmetics are often applied to large skin surfaces, significant amounts of sunscreens could enter the systemic circulation, causing adverse reactions, even at low penetration rates (4,5). Many papers (6,7) deal with phototoxicity and photoallergenicity induced by topical use of sunscreen agents, and Knowland et al. (8) reported that octyl dimethyl PABA induced mutagenicity in yeast cells after exposure to UV irradiations in vitro. However, little has been published about sunscreen chronic toxicity and disposition after topical application, although some pa- pers suggest that certain UV filters could be absorbed through the skin. Hayden et al. (5) reported that after topical application of a sunscreen formulation containing oxy- benzone on human volunteers, about 1-2% of the sunscreen was absorbed through the skin over a 10-h period. In vitro skin permeation experiments performed using different formulations containing 5% w/w octyl salicylate showed that the percutaneous absorp- tion of this sunscreen ranged from 0.23% to 0.65% of the applied dose, depending on the formulation tested (9). The evaluation of some commercial sunscreen products containing different UV filters showed that, among the sunscreen agents tested, ben- zophenone-3 permeated the skin in significant amounts (10% of the applied dose) while the other UV filters penetrated into the skin without passing into the blood stream (10). Therefore, percutaneous absorption studies could provide useful information about the potential toxicity of sunscreen agents. As widely reported in the literature (11-13), the ability of a molecule to penetrate the skin depends mainly on both its physicochemical properties and vehicle composition (14,15). In vitro skin penetration studies on two sunscreen agents (benzophenone-3 and octylmethoxycinnamate) from topical formulations showed that their stratum corneum retention and their penetration basically depended on the vehicle composition (16). In vitro skin permeation experiments on oxybenzone, 2-ethylhexyl-4-methoxycinnamate, and 2-ethylsalicylate from O/W emulsion-gel and petroleum jelly showed that the vehicle strongly affected UV-filter penetration into the different skin layers (17). Simi- larly, other authors (18) showed that different benzophenone derivatives permeated the skin in different amounts, depending on the vehicle used to incorporate the sunscreen agent. One of the most important requirements for sunscreen products is their water resistance, which often has been increased through the use of silicones (19,20). Silicone derivatives have recently been evaluated as emulsifiers to obtain topical products (21) since they show some advantages compared to conventional surfactants: (a) they are stable even at high temperature, (b) water/silicone emulsions (W/S) can easily be obtained at room temperature without vigorous stirring, and (c) W/S emulsions feel as good on the skin as O/W emulsions and have a high water resistance similar to that of W/O emulsions (22). Hydrocarbon emulsifiers have been reported to affect active compound skin permeation, depending on the vehicle composition and on the physicochemical properties of both the surface-active compound and the active ingredient (23,24), while the effects of silicone emulsifiers on the percutaneous absorption process have been poorly studied. The aim of this work was to investigate the effects of some silicone emulsifiers on in vitro permeation through human skin of two of the most commonly used sunscreens agents, octylmethoxycinnamate (OMC) and butylmethoxybenzoylmethane (BMBM). Therefore, W/S cosmetic emulsions were prepared using silicone emulsifiers containing different
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)