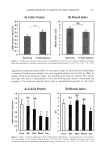
SILICONE EMULSIFIERS AND SUNSCREEN PERMEATION 511 alkyl groups or a combination of cyclomethicone and dimethicone copolyol, and their influence on in vitro skin permeation and the release rates of OMC and BMBM were evaluated. MATERIALS AND METHODS MATERIALS Cyclopentasiloxane and dimethicone/vinyldimethicone crosspolymer (DC 9040©), cy- clomethicone and dimethicone copolyol (DC 3225C©), and lauryldimethicone copolyol (DC 5200©), were a kind gift from Dow Corning Corporation (Milan, Italy). Cyclome- thicone (SF 1202©), methylchloroisothiazolinone and methylisothiazolinone (Kathon CG ©) and imidazolidinyl urea (Gram 1 ©) were kindly supplied by Sinerga S.r.L. (Milan, Italy). Dimethicone copolyol and cyclomethicone (Abil EM 97©), polyglyceryl-4- isostearate and cetyldimethicone copolyol and hexyllaurate (Abil WE 09©), methylglu- cose dioleate (Isolan DO©), and cetyldimethicone copolyol (Abil EM 90 ©) were pur- chased from Th. Goldschmidt Ag (Milan, Italy). Octylmethoxycinnamate (OMC) (Uvinul MC 80 ©) and butylmethoxydibenzoylmethane (BMBM) (Uvinul BMBM ©) were a kind gift from BASF (Germany). Glycerin and disodium EDTA were obtained from Galeno (Milan, Italy). Sodium chloride was a Carlo Erba Reagenti product (Milan, Italy). Perfume (Mamba) was a gift from Muller and Koster (Italy). Cellulose acetate mem- branes (Spectra/Por CE mol. wt. cut-off 3.000) were bought from Spectrum (Los An- geles, CA). Acetonitrile and water used in the HPLC procedures were of LC grade and were purchased from Merck (Germany). EMULSION PREPARATION The composition of the tested emulsions is reported in Tables I and II. The oil phase components (phase A), apart from the sunscreen agents, were placed in a glass container and stirred to obtain a homogeneous mixture. The sunscreens were then added and the Table I Composition of Emulsions 1-5 Ingredients % w/w Phase A SF 1202 15.0 Isolan DO 0.5 DC 9040 5.0 OMC 5.0 BMBM 0.5 Emulsifier q.b. Phase B Glycerol 5.0 Kathon CG 0.05 Gram 1 0.35 Disodium EDTA 0.1 Sodium chloride 1.0 Water q.b. 100.0
512 JOURNAL OF COSMETIC SCIENCE Table II Silicone Emulsifiers (% w/w) Used to Prepare Emulsions 1-5 Formulation Emulsifier % w/w 1 Abil EM 97 2.5 2 Abil EM 90 2.5 3 Abil WE 09 5.0 4 DC 52OO 2.5 5 DC 3225C 10.0 mixture was gently stirred. The aqueous phase was added to the oil phase drop by drop under continuous stirring. The pre-emulsion thus formed was vigorously stirred by means of a turbomixer (Silverson SL2) for 2 min to obtain an emulsion. All the formu- lations were stored at room temperature in airtight glass containers until used. VISCOSITY AND pH MEASUREMENTS The viscosity and pH of emulsions 1-5 were determined 48 h after their preparation. The viscosity (mPas) was determined with a Mettier Rheomat RM 260 viscosimeter using a MSDIN 125 spindle at shear rate 3 for 10 sec. The instrument was connected to a computer IBM PS/2 70. SWR 37 V 2.20 software was used for data elaboration. Samples of the emulsions were left to settle for 30 min at room temperature before starting the analyses. A Crison pH meter, model Basic 20, was used to measure the pH values of emulsions 1-5 at room temperature. IN VITRO PERMEATION EXPERIMENTS THROUGH HUMAN SKIN Samples of healthy adult human skin (mean age 35 + 8 years) were obtained from abdominal reduction surgery. Membranes of stratum corneum and epidermis (SCE) were prepared as previously described (25). Briefly, subcutaneous fat was removed and the skin was immersed in distilled water at 60 ø + iøC for 2 min. The stratum comeurn and the epidermis (SCE) were then peeled off, dried in a desiccator at approximately 25% relative humidity, wrapped in aluminum foil, and stored at 4 ø + iøC (26). SCE mem- branes were used since other authors (27) reported that the dermis can act as an addi- tional artificial barrier when in vitro skin permeation of lipophilic compounds, such as sunscreens, is investigated. The SCE membranes were rehydrated by immersion in distilled water for 1 h at room temperature before being mounted in Franz-type diffusion cells (LGA, Berkeley, CA). The cells had a diffusion surface area of 0.75 cm 2 and a 4.5-ml receiving chamber volume. The receptor was filled with water/ethanol (50:50) to ensure pseudo-sink conditions by increasing sunscreen solubility in the receiving phase, as previously reported in the literature (28,29). The receptor fluid was constantly stirred and thermostated at 37øC. Each formulation (200 mg) was applied on the skin surface, and the experiment was run for 22 h. Due to sunscreen photoinstability, all experiments were carried out avoiding light exposure. At the end of the experiments the receptor phase was withdrawn and analyzed by the HPLC method described below to determine the total amount of permeated sunscreen. Each experiment was carried out in duplicate
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)