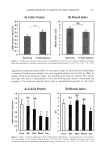
SALICYLIC ACID DELIVERY 529 25 • 2o • 10 .•_ 5 0 L G MO DMI B T PG Figure 8. Follicular delivery from Type II formulations. *Statistically significant at the 95% confidence level, two-tailed t-test compares the formulation without polar vehicle with the formulation containing the polar vehicle. Error bars indicate the standard error of the mean of six readings. quantity of SA delivered, as compared to the control in which no polar vehicle other than water was added. The exception to this rule was the propylene glycol formulation. This can be explained by the fact that all these formulations have the same oil phase, namely, L. The partitioning of the drug is not affected by the addition of a polar vehicle to the water phase. It seems the type of oil phase affects follicular delivery to a higher degree than the polar or water phase. The addition of PG to the formulation caused a significant increase in the follicular deposition of SA as compared to the control. It is possible that propylene glycol and Labrafil have a synergistic effect. The presence of Labrafil in the PG formulation caused better delivery than the Labrafil formulation or neat PG. Propylene glycol in combination with oleic acid also has been shown to have a better permeability coefficient than PG alone (16). The synergistic action of PG with IPM was also seen in a study that investigated in vitro penetration of diclofenac sodium through a synthetic membrane. In a study that investigated the permeation of nifidipine across hairless mouse skin, a similar enhancement in flux was found for PG with OA and DMI as compared to these vehicles used individually (17). It is possible that PG can also act in a synergistic manner with L for enhanced follicular drug delivery. It is important to note here that if we were to make formulations using these other polar vehicles, there would be no significant decrease in the percent of SA delivered in the sebaceous glands. This would mean that if we had to use these polar vehicles in the formulations for follicular delivery, their presence would not affect the delivery of the drug. Thus lipophilic vehicles are miscible with sebum and therefore increase the delivery of SA. In the case of hydrophilic products, SA partitions from the vehicle into sebum and the driving force (by equal concentration of drug in the formulations) is increased if the maximum solubility is decreased (increased thermodynamic activity). CONCLUSIONS From the results above it can be seen that trends exist between the DSC data and the follicular delivery results for SA, depending on the type of vehicle used. Hence DSC
530 JOURNAL OF COSMETIC SCIENCE might be useful as a screening tool to identify a vehicle that could be miscible with sebum and aid in the delivery of the drug (SA) into the follicles. Furthermore, vehicles that aid in the delivery of SA to the sebaceous glands have also been identified. These results suggest that, depending on the polarity of the vehicle and the physicochemical properties of the drug, delivery to the sebaceous glands takes place mainly by two different mechanisms. The results given above may help to optimize formulations for follicular drug delivery. Further studies with other molecules and more vehicles to get a better insight into the mechanism by which the vehicles can control delivery are suggested. For both types of formulation, the rank order of the neat polar vehicles was not neces- sarily followed by their respective formulations. It is possible that many interactions occur between the oil phase and the polar phase, and this may lead to changes that affect follicular delivery. Moreover, the formulations contain the surfactant Tween 80, which may affect follicular delivery. To increase follicular delivery we would probably have to increase the oil phase volume and in particular use either OA or IPM. Also, the addition of PG to the formulations may cause a synergistic affect, as it did with Labrafil. Further suggestions to increase follicular delivery would be to use oil as an external phase in the emulsion, rather than as the internal phase. Use of an appropriate oil phase would further enhance follicular delivery of SA, as shown by our study on neat vehicles. In conclusion, we have gained a better insight into the mechanism of follicular delivery of SA using neat vehicles and emulsion formulations. REFERENCES (1) J. S. Strauss, P. E. Pochi, and D. T. Downing, The sebaceous glands: Twenty five years of progress, J. Invest. Dermatol., 67, 90-97 (1976). (2) M.R. Motwani, L.D. Rhein, and J. L. Zatz, Differential scanning calorimetry studies of sebum models, J. Cosmet. Sci., 52, 211-224 (2001). (3) M. R. Motwani, L. D. Rhein, and J. L. Zatz, Influence of vehicles on the phase transitions of model sebum, J. Cosmet. Sd., 53, 35-42 (2002). (4) L.M. Lieb, C. Ramachandran, K. Egbaria, and N. Weiner, Topical delivery enhancement with multilamellar liposomes into pilosebaceous units. I. In vitro evaluation using fluorescent techniques with the hamster ear model, J. Invest. Dermatol., 99, 108-113 (1992). (5) S.C. Jayaraman, Follicular delivery of erythromycin from nonionic liposomes and emulsions (Doctoral dissertation, University of Michigan, 1997). (6) L. M. Lieb, G. L. Flynn, and N. D. Weiner, Follicular (pilosebaceous unit) deposition and pharmaco- logical behavior of cimetidine as a function of formulation, Pharmaceut. Res., 11, 1414-1423 (1994). (7) S. Niemiec, C. Ramachandran, and N. D. Weiner, Influence of nonionic liposomal composition on topical delivery of peptide drugs into pilosebaceous units: An in vivo study using the hamster ear model, Pharmaceut. Res., 12, 1184-1188 (1995). (8) N. D. Weiner, Targeted delivery of macromolecules via liposomes, Int. J. Pharmaceut., 162, 29-38 (1998). (9) S. M. Niemiec, C. Ramachandran, and N. D. Weiner, Perifollicular transgenic expression of human interleukin-1 receptor antagonist protein following topical application of novel liposome-plasmid DNA formulations in vivo, J. Pharmaceut. Sci., 86, 701-708 (1997). (10) E.G. Weirich, J. K. Longauer, and A. H. Kirkwood, Dermatopharmacology of salicylic acid, Derma- tologica, 151, 321-332 (1975). (11) K.M. Norclstrom, J. N. Labows, K.J. McGinley, and J.J. Leyden, Characterization of wax esters, triglycerides, and free fatty acids of follicular casts, J. Invest. Dermatol., 86, 700-705 (1986). (12) L. M. Lieb, Formulation factors affecting follicular (pilosebaceous route) drug delivery as evaluated with hamster ear model (Doctoral dissertation, University of Michigan, 1994).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)