SILICONE EMULSIFIERS AND SUNSCREEN PERMEATION 513 on skin samples from three different donors. The results are expressed as the cumulative amount and percentage of the applied dose of permeated sunscreen after 22 h. IN VITRO RELEASE EXPERIMENTS Sunscreen release rates from emulsions 1-5 were measured through cellulose acetate membranes by means of the same Franz diffusion cells described above. This technique has been previously reported in the literature as a suitable method for evaluating active compound release from topical formulations (30). Release experiments were carried out using the same experimental conditions described for in vitro skin permeation experiments. Two hundred milligrams of each formulation were placed on the membrane surface previously moistened with the receptor phase (water/ethanol 50:50). Samples of the receptor phase (200 121) were withdrawn at intervals and replaced with an equal volume of receiving solution equilibrated to the experimental temperature (3 5 øC). The samples of the receptor phase were analyzed by the HPLC method described below to determine their sunscreen content. Each experiment was run for 22 h and performed in triplicate. ANALYTICAL DETERMINATION OF SUNSCREENS OMC and BMBM concentrations in the receiving compartment of Franz cells were determined by HPLC analysis. A Varian model 9010 liquid chromatographic system (Varian, Milan, Italy) equipped with a model 9020 UV-Vis detector, a 20-pl Rheodyne model 7125 injection valve, and a Varian 4010 recorder was used. The chromatographic analyses were carried out with a Waters Simmetry 4.6 x 15-cm reverse-phase column (C•8) at room temperature. The mobile phase was an acetonitrile/water mixture (80:20 v/v) with a flow rate of 1 ml/min. The column effluent was monitored continuously at 310 nm to detect OMC and at 360 nm to detect BMBM. The amounts of OMC and BMBM were calculated by reporting the peak area of a sample on a standard calibration curve that was built up by relating known OMC and BMBM concentrations to the respective peak areas. Standard solutions contained between 0.8 and 16 pg/ml of OMC 2 and BMBM. Linear regression analysis of the peak areas of standard solutions gave a r value of 0.9998. No interference of the other formulation components was observed. The sensitivity of the HPLC method was 0.1 pg/ml for both sunscreens. STATISTICAL ANALYSIS Results are expressed as the mean value + the standard deviation (SD). Statistical data analysis was performed using a one-way ANOVA with a subsequent Bonferroni t-test. Data with p 0.05 are considered significant. RESULTS AND DISCUSSION Silicone emulsifiers have been recently used to formulate sunscreen cosmetic emulsions due to their very low toxicity after topical application and their ability to impart to the
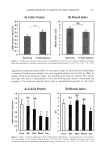
514 JOURNAL OF COSMETIC SCIENCE products a noticeable water resistance (21,22). In order to evaluate the effects of some silicone emulsifiers on in vitro release and skin permeation of two sunscreen agents (OMC and BMBM), we prepared five emulsions containing different silicone surfactants. The viscosity and pH values of emulsions 1-5 are reported in Table III. All the tested formulations showed similar pH values (4.4-5.0), while their viscosity values were strongly influenced by the emulsifier system used and decreased in the order 1 2 4 3 5. Since both the oily and the aqueous phase had the same composition, the different viscosity values of these formulations might be due to different interactions among the silicone surfactants, the cross-polymer used as thickening agent, and the aqueous and oily phases. The results of in vitro OMC and BMBM skin permeation experiments from emulsions 1-5 are reported in Table IV. The results are expressed in terms of the cumulative amount permeated after 22 h because the sensitivity of the analytical method did not allow the detection of OMC and BMBM in the receiving phase until 8-10 h from the beginning of the experiment and no flux could be calculated under these conditions. OMC in vitro skin permeation from formulations 1-5 decreased in the order 2 • 1 5 3 • 4 and a twofold increase was observed when comparing emulsion 2 with emulsion 4. The cumulative amount of BMBM permeated through human skin after 22 h was not significantly different with emulsions 1-5. As regards OMC, the two different combi- nations of dimethicone copolyol and cyclomethicone used to prepare emulsions 1 and 5 did not significantly affect OMC skin permeation, while the use of silicone emulsifiers with a different alkyl chain length influenced OMC skin permeation (emulsions 2 and 4). As reported in the literature (24), the structure of nonionic surfactants may affect the skin penetration of drugs and the size and the shape of both the alkyl chain and the polar head group of the emulsifier could be regarded as important factors in determining their ability to enhance skin permeation. A similar influence of the alkyl chain length was observed in our experiments, since OMC skin permeation was different with formula- tions containing silicone emulsifiers with the lauryl or cetyl alkyl group. However, since this effect was observed only for OMC, other factors could be taken into account to explain the different skin permeation of these two sunscreens. As shown in Table IV, although the cumulative amount of permeated BMBM was lower than that of OMC, the percentage of the permeated applied dose was similar for both UV filters. Therefore, the different percentages of sunscreens used to prepare emulsions 1-5 (OMC 5% w/w BMBM 0.5% w/w) could not account for the lower skin permeation of BMBM. The different skin permeation profiles of OMC and BMBM obtained from emulsions Table III Viscosity and pH Values of Emulsions 1-5 Viscosity Formulation pH (mPas) 4.4 22.9OO 4.5 13.400 5.0 4.9OO 4.4 13.000 4.7 3.700
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)