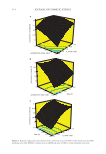
374 JOURNAL OF COSMETIC SCIENCE A B C 1.00 1.25 1.50 1.75 2.00 0.25 0.38 0.50 0.63 0.75 47 57.25 67.5 77.75 88 acid/alcohol molar ratio IL molar ratio 1.00 1.25 1.50 1.75 2.00 4.00 5.00 6.00 7.00 8.00 65 70.75 76.5 82.25 88 acid/alcohol molar ratio time (h) 0.25 0.38 0.50 0.63 0.75 4.00 5.00 6.00 7.00 8.00 61 68.25 75.5 82.75 90 IL molar ratio time (h) Figure 8. Response surface plots and contours of the conversion of acid. (A) Effect of the molar ratio of AA:BA and molar ratio of IL, (B) Effect of molar ratio of AA:BA and time, (C) Effect of time and molar ratio of IL. %) Convesonofacd %) Convesonofacd %) onesonofacd C
375 SYNTHESIS OF BENZYL ACETATE highest conversion, was investigated in benzyl acetate esterification reaction. The exper- iments were carried out with AA:BA:[EMIM] [HSO 4 ] molar ratio of 1:1:075 at 110° C for 8 hours. At the end of the reaction, the upper phase and the lower phase (IL and water) were separated by simply decantation. The IL was dried under vacuum (0.01 Torr) at 100° C (overnight). The reusability of the IL was investigated by repeating the same experimental procedure five times for the recycled IL. Figure 7 represents that the cata- lytic activity of [EMIM] [HSO 4 ] did not change until the third run, but began to decrease slightly with the next use. CONCLUSIONS In this study, the obtaining of benzyl acetate, which gives artificial jasmine scent and apple, banana flavors to various cosmetics and personal care products like perfumes, lotions, hair creams, and perfumes, by esterification method was investigated. For this purpose, five different ILs were used as a catalyst for the esterification reaction of AA with BA. The results showed that the highest acid conversion was obtained in the ester- ification reaction, in which [EMIM] [HS0 4 ] was used as a catalyst. The response surface methodology based on the Box–Behnken design for optimizing the reaction parameters was applied using this IL. The optimum conditions were AA:BA molar ratio of 1:1, IL molar ratio of 0.66, and reaction time of 4 h. Under these conditions, 90.34% acid conversion was achieved. ANOVA was performed to test the suitability of the proposed second-degree model in order to determine the effect levels of the independent variables affecting the acid conversion. According to the statistical results obtained, it was seen that the most crucial factor affecting the reaction was the amount of IL. As a result, the IL used in this study proved to be an excellent catalyst for the esterification of AA and BA. This study has been a green approach obtaining the ester with high efficiency, and easy separation and recovery of the IL used as a catalyst from the product. In future research, it is important to study that different esters, which are frequently used in personal care products, can also be obtained using this method. ACKNOWLEDGMENTS This study was funded by Istanbul University with Project Number 34009. REFERENCES (1) D. McGinty, D. Vitale, C. S. Letizia, and A. M. Api, Fragrance material review on benzyl acetate, Food Chem Toxicol., 50, 363–384 (2012). (2) R, Lamba, S. Kumar, and S. Sarkar, Esterification of decanoic acid with methanol using Amberlyst 15: reaction kinetics, Chem Eng Commun., 205, 281–294 (2018). (3) J. Lilja, J. Aumo, T. Salmi, D. Y. Murzin, P. M. Arvela, M. Sundell, K. Ekman, R. Peltonen, and H. Vainio, Kinetics of esterification of propanoic acid with methanol over a fibrous polymer-supported sulphonic acid catalyst, Appl Catal A-Gen., 228, 253–267 (2002). (4) H. J. Bart, J. ReIdetschlager, K. Schatkaj, and A. Lehmann, Kinetics of esterification of levulinic acid with n-butanol by homogeneous catalysis, Ind Eng Chem Res., 33, 21–25 (1994) (5) J. Lilja, D. Y. Murzin, T. Salmi, J. Aumo, P. Maki-Arvela, and M. Sundell, Esterification of different acids over heterogeneous and homogeneous catalysts and correlation with the Taft equation, J Mol Catal-A Chem, 182, 555–563 (2002).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)