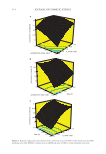
415 THE EFFECT OF VEGETABLE OIL COMPOSITION properties was the fatty acid chain length composition of the oil, more precisely the per- centage of fatty acid chain with a length of 18 carbons. Above 90% of fatty acid chain with a length of 18 carbons in the oil, the optimal ratio was 7:3, below 90% the optimal ratio was 8:2. In the literature, the fatty acid chain length in the oil is described as possi- ble parameter modifying the orientation of the platelet crystals inside the liquid oil giv- ing rise to different rheological properties (39). The different orientation of the platelet crystals formed by the oleogelator system could lead to a modification of the distribution of the crystalline material inside the oil, resulting in different rheological properties as a function of the oil. To verify this hypothesis, complementary measurements would be necessary in order to use the model developed by Miyazaki and Marangoni based on the cellular solid approach of Gibson and Ashby (47,48). This model was successfully applied to different oleogels to explain the relationship between the mechanical properties of oleogels and their microstructure (20,48). As perspective also to continue this work, it would be interesting to study the effect of minor polar compounds of each oil by removing them as described recently by Scharfe et al. (37). By removing these polar compounds, it would be possible to understand their effect on the BO/BA oleogelator system in terms of oleogel structures and properties (38). Our results obtained with cosmetic grade raw materials have practical applications for the cosmetic industry since it shows how to obtain the best oleogels in terms of texture and stability, and then to use them to produce oil foams (11,40). As a function of the fatty acid chain length composition of the oil, the ratio between BO and BA can be adjusted in order to tune the oleogel properties. ACKNOWLEDGMENTS This research was supported by L’OREAL R&I. REFERENCES (1) E. D. Co and A. G. Marangoni, Organogels: an alternative edible oil-structuring method, J. Am. Oil. Chem. Soc., 89, 749–780 (2012). (2) A.G. Marangoni and N. Garti, Edible Oleogels: Structure and Health Implications (AOC Press, San Diego, CA, 2018). (3) M. Suzuki and K. Hanabusa, Polymer organogelators that make supramolecular organogels through physical cross-linking and self-assembly, Chem. Soc. Rev., 39, 455–463 (2010). (4) T. Dürrschmidt and H. Hoffmann, Organogels from ABA triblock copolymers, Colloid. Polym. Sci., 279, 1005–1012 (2001). (5) M. Davidovich-Pinhas, S. Barbut, and A. G. Marangoni, The gelation of oil using ethyl cellulose, Car- bohydr. Polym., 117, 869–878 (2015). (6) M. Hermansson, The fluidity of hydrocarbon regions in organo-gels, studied by NMR: basic trans- lational and rotational diffusion measurements, Colloids Surfaces A Physicochem, Eng. Asp., 154, 303–309 (1999). (7) M. A. Rogers and R. G. Weiss, Systematic modifications of alkane-based molecular gelators and the consequences to the structures and properties of their gels, New. J. Chem., 39, 785–799 (2015). (8) R.G. Weiss and P. Terech, Molecular Gels, Kluwer Aca (Kluwer Academic Publishers, Dordrecht, the Netherlands, 2006). (9) M. Zhang and R.G. Weiss, Self-assembled networks and molecular gels derived from long-chain, naturally-occurring fatty acids, J. Braz. Chem. Soc., 27, 239–255 (2016). (10) A.-L. Fameau and M.A. Rogers, The curious case of 12-hydroxystearic acid–the Dr. Jekyll & Mr. Hyde of molecular gelators, Curr. Opin. Colloid Interface Sci., 45, 68–82 (2020).
416 JOURNAL OF COSMETIC SCIENCE (11) R. M. Martinez, C. Rosado, M. V. R. Velasco, S. C. da S. Lannes, and A. R. Baby, Main features and applications of organogels in cosmetics, Int. J. Cosmet. Sci., 41, 109–117 (2019). (12) M. Samateh, S. S. Sagiri, and G. John, in A.G. Marangoni & N. Gharti Molecular Oleogels: Green Approach in Structuring Vegetable Oils (Elsevier, Edible Oleogels, 2018), pp. 415–438. (13) A. R. Patel, “Shellac-based oleogels,” in Edible Oleogels, A. G. Marangoni and N. Garti. Eds. (AOC Press, San Diego, CA, 2018), pp. 173–192. (14) E. Scholten, Edible oleogels: how suitable are proteins as a structurant?, Curr. Opin. Food Sci., 27, 36–42 (2019). (15) A. I. Romoscanu and R. Mezzenga, Emulsion-templated fully reversible protein-in-oil gels, Langmuir., 22, 7812–7818 (2006). (16) A. Bot and E. Flöter, “Structuring edible oil phases with fatty acids and alcohols,” in Edible Oil Struct, A.G. Marangoni and N. Garti. Eds. (AOC Press, San Diego, CA, 2018), pp. 95–105. (17) M. Eini and D. Tamarkin, Pharmaceutical and cosmetic carrier or composition for topical application, Vyne Pharmaceuticals Ltd, U.S. Patent No. 6,967,023, 2005. (18) F.G. Gandolfo, A. Bot, and E. Flöter, Structuring of edible oils by long-chain FA, fatty alcohols, and their mixtures, J. Am. Oil Chem. Soc., 81, 1–6 (2004). (19) H. M. Schaink, K. F. van Malssen, S. Morgado-Alves, D. Kalnin, and E. van der Linden, Crystal network for edible oil organogels: possibilities and limitations of the fatty acid and fatty alcohol systems, Food. Res. Int., 40, 1185–1193 (2007). (20) C. Blach, A. J. Gravelle, F. Peyronel, J. Weiss, S. Barbut, and A. G. Marangoni, Revisiting the crystal- lization behavior of stearyl alcohol: stearic acid (SO: SA) mixtures in edible oil, Rsc. Adv., 6, 81151– 81163 (2016). (21) A. J. Gravelle and A. G. Marangoni, “Edible oleogels,” in Vegetable Oil Oleogels Structured Using Mixtures of Stearyl Alcohol and Stearic Acid (SO: SA) in Edible Oleogels, A. G. Marangoni and N. Garti. Eds. (AOC Press, San Diego, CA, 2018), (Elsevier, 2018) , pp. 193–217. (22) H.M. Schaink, The solid–liquid phase diagram of binary mixtures dissolved in an inert oil: application to ternary blends that can form organogels, J. Am. Oil Chem. Soc., 97, 117–124 (2020). (23) F. Valoppi, S. Calligaris, and A. G. Marangoni,” Edible oleogels,” in Stearyl Alcohol Oleogels (Elsevier, 2018), pp. 219–234. (24) A. R. Patel and K. Dewettinck, Edible oil structuring: an overview and recent updates, Food. Funct., 7, 20–29 (2016). (25) F. C. Wang, A. J. Gravelle, A. I. Blake, and A. G. Marangoni, Novel trans fat replacement strategies, Curr. Opin. Food Sci., 7, 27–34 (2016). (26) F. Valoppi, S. Calligaris, and A. G. Marangoni, Structure and physical properties of oleogels containing peanut oil and saturated fatty alcohols, Eur. J. Lipid Sci. Technol., 119, 1600252 (2017). (27) M. Callau, K. Sow-Kébé, L. Nicolas-Morgantini, and A. L. Fameau, Effect of the ratio between òhol and behenic acid on the oleogel properties, J. Colloid. Interface. Sci., 560, 874–884 (2020). (28) Y. Lan, M. G. Corradini, aR. G. Weiss, S. R. Raghavan, and M. A. Rogers, To gel or not to gel: correlat- ing molecular gelation with solvent parameters, Chem. Soc. Rev., 44, 6035–6058 (2015). (29) S. Wu, J. Gao, T. J. Emge, M. A. Rogers, Influence of solvent on the supramolecular architectures in molecular gels, Soft. Matter., 9, 5942–5950 (2013). (30) M. Raynal and L. Bouteiller, Organogel formation rationalized by Hansen solubility parameters, Chem. Commun., 47, 8271–8273 (2011). (31) J. Bonnet, G. Suissa, M. Raynal, and L. Bouteiller, Organogel formation rationalized by Hansen solu- bility parameters: dos and don’ts, Soft. Matter., 10, 3154–3160 (2014). (32) D.R. Nunes, M. Raynal, B. Isare, P.-A. Albouy, and L. Bouteiller, Organogel formation rationalized by Hansen solubility parameters: improved methodology, Soft. Matter., 14, 4805–4809 (2018). (33) T. Laredo, S. Barbut, and A.G. Marangoni, Molecular interactions of polymer oleogelation, Soft. Matter., 7, 2734–2743 (2011). (34) H. Sawalha, G. Margry, R. den Adel, P. Venema, A. Bot, E. Flöter, and E. van der Linden, The influence of the type of oil phase on the self‐assembly process of γ‐oryzanol+ β‐sitosterol tubules in organogel systems, Eur. J. Lipid Sci. Technol., 115, 295–300 (2013). (35) S. Calligaris, G. Mirolo, S. Da Pieve, G. Arrighetti, and M. C. Nicoli, Effect of oil type on forma- tion, structure and thermal properties of γ-oryzanol and β-sitosterol-based organogels, Food. Biophys., 9, 69–75 (2014).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)