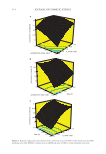
409 THE EFFECT OF VEGETABLE OIL COMPOSITION crystalline particles species were present for these ratios with different melting and crystalli- zation temperatures (19,20). The same behavior was observed that has previously been seen for the sunflower oil (27). For all the oleogels, the evolution of the melting peak was plotted as a function of R (Figure 3). All the oils exhibited the same evolution as a function of R (Table SI.3). For R = 10:0, the melting temperature was around 55°C. The lowest melting temperature was reached for R = 8:2. By increasing R, an increase of the melting temperature was observed and reached a maximal value around 62°C for R = 0:10. For the total enthalpy of the endotherm, we observed the same trend for all oleogels: a decrease of the total enthalpy from R = 10:0 to R = 3:7 and then an increase until R = 0:10 (Table SI.3). When we com- pared these results with those obtained for stearyl alcohol/stearic acid based oleogels, we observed the same trend (42). This variation of enthalpy as a function of R was attributed to the formation of mixed crystals in the systems (16). For the thermal behavior of the oleogels, no oil effect was distinguished (Table SI.3). All the oleogels had the same behavior. EFFECT OF THE OIL ON THE CRYSTAL STRUCTURE To determine the crystals’ nature (pure BO, pure BA, or mixed BO/BA) for each oleogel as a function of both R and the nature of the oil, Small and Wide-Angle X-ray scattering (SAXS and WAXS, respectively) measurements were performed at 25°C. From the SAXS regime, information about long spacing linked to chain length, chain tilt and number of chains per layer can be obtained. From the WAXS regime, information related to the short spacing can be obtained corresponding to the lateral packing of the chains. In Fig- ure 4, all the spectra in the SAXS and WAXS regimes are shown. All the d-spacings are shown in Table II with the nature of crystalline structure. In the SAXS regime, for R = 10:0 containing only BO, for all the oils, one main peak was detected corresponding to a d-spacing of around 57.1 Å and another additional peak with a d-spacing of around 48.3 Å (Figure 4A). Each of these peaks was followed by additional higher order reflections peak visible on the spectrum. The presence of these two long d-spacings indicated the coexistence of two different packing arrange- ments as already reported by Valoppi et al. for fatty alcohols in peanut oil (43). Based on the literature, it is supposed that some of BO molecules were crystallized in a double layer structure associated with d = 57.1 Å (43). The other BO molecules were organized into a triple layer structure with a d-spacing around 100 Å that can be deduced from the position of the second order reflection peak observed at d = 48.3 Å (19). In the WAXS region for all the oils, six peaks were detected, which were almost at the same position except a slight shift for the rapeseed oil (Figure 4B). We suppose that these peaks can be ascribed to two polymorphic forms, the β’ and γ-form as described for fatty alcohols in peanut oil (43). For all the oleogels containing only BA (R = 0:10), only one main peak followed by its higher order reflections was detected corresponding to a d-spacing of 48.3 Å. We suppose that BA molecules were crystallized in a double layer structure associated with d = 48.3 Å. Other addi- tional peaks appeared on the SAXS curves coming from some polymorphic structures present inside the oleogels at low concentration. In the WAXS regime, five peaks were detected corre- sponding to the main crystalline structure present inside the oleogel (Table II). By comparing this result with the literature, we supposed that BA in the oleogels showed only one molecu- lar packing arrangement and crystallized into the most stable C form with a monoclinic unit cell (44).
410 JOURNAL OF COSMETIC SCIENCE For R = 8:2, R = 7:3, and R = 5:5, all the oleogels exhibited the same SAXS and WAXS scattering profiles (Figure 4A and B). In the SAXS regime, only one main peak followed by its higher order reflections was detected. This peak gave a d-spacing of 57.1 Å. In the WAXS regime, six peaks were located at the same position whatever the oil used (4.6, 4.5, 4.0, 3.8, 3.6, and 3.5 Å). The d-spacing for the SAXS and WAXS regime could not be associated to the d-spacings measured for oleogels containing only BO (R = 10:0) or only BA (R = 0:10). This observation is in line with the results obtained by Blach et al. on oleogel based on stearic acid/stearyl alcohol (20). Therefore, only one type of crystal was present in those systems: mixed crystals of BO and BA. The two components co-crystal- lized to give only one crystalline structure as already observed in oleogels based on stearyl alcohol/stearic acid (19,20,22). When the concentration of BA was higher than the concentration of BO (R = 3:7 and R = 2:8), the scattering spectra were similar for all oils. In the SAXS regime, for both R = 3:7 and R = 2:8, the peak corresponding to the d-spacing of 57.0 Å observed pre- viously for R = 8:2, R = 7:3 and R = 5:5 remained. Mixed crystals were also present for these two ratios. Furthermore, another peak appeared on the spectra associated with a d-spacing of 48.3 Å corresponding to the d-spacing found for oleogels containing pure BA (R = 0:10). These two oleogels (R = 3:7 and R = 2:8) were then composed of mixed crystals of BO/BA and of pure BA crystals. The WAXS spectra confirmed this Figure 4. (A) SAXS and (B) WAXS spectra of oleogels with varying ratios of behenyl alcohol:behenic acid (BO:BA), with a total of 10 wt.% oleogelator in various oils: olive, apricot, camelina, and rapeseed. The mea- surements were carried out at 25°C. The spectra were shifted in intensity for clarity.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)