399 Address all correspondence to Anne-Laure Fameau at anne-laure.fameau@inrae.fr. J. Cosmet. Sci., 72, 399–417 ( July/August 2021) The Effect of Vegetable Oil Composition on the Structural Properties of Oleogels Based on Behenyl Alcohol/Behenic Acid Oleogelator System MARION CALLAU, NINA JENKINS, KOUDEDII SOW-KEBE, CLEMENT LEVIVIER, and ANNE-LAURE FAMEAU, L’Oréal Research and Innovation, 93000 Saint-Ouen, France (M.C., N.J., K.S., C.L., A.F.). Accepted for publication May 27, 2021. Synopsis Recently, we described that the weight ratio (R) between behenyl alcohol (BO) and behenic acid (BA) in sunflower oil effects the textural and structural properties of the oleogel system. One R (7:3) was found as optimal since it led to an enhancement of the oleogel properties for both the hardness and the stability in terms of oil-binding capacity. However, what remains unknown is the effect of other vegetable oils. There- fore, in this study, we aim to test a range of different vegetable oils that are widely used in the cosmetic industry. All the oleogels were prepared by heating together at 85°C the oil and the fatty components under magnetic stirring. After heating, the samples were allowed to cool down quiescently to room tem- perature without any stirring. The oil properties tested included viscosity, density, and surface tension. The oleogel properties (hardness, oil loss, and gel stability) and their structure as a function of R were characterized at different length scales by coupling optical microscopy, differential scanning calorimetry (DSC), Small-Angle X-ray Scattering (SAXS), and Wide-Angle X-ray Scattering (WAXS) experiments. The same crystal structure evolution determined by SAXS and WAXS as a function of R was observed whatever the oil. In the DSC profiles and optical microscopy pictures, no oil effect was detected. However, our results highlighted two different optimal ratios, giving rise to the best oleogels in terms of stability (oil loss) and hardness as a function of the oil. For sunflower, apricot, and rapeseed oils, R = 7:3 was the optimal ratio, whereas R = 8:2 was the optimal ratio for olive and camelina oil. These observations were correlated with the fatty acid chain length composition of the oil. The results obtained have practical applications for the cosmetic industry since it establishes formulation rules for oleogel systems. Oleogels are based on BO and BA components, which are raw materials widely used for hair and skin applications. Different oils have different fatty acid chain lengths composition and as a result, the ratio between BO and BA needs to be adjusted in order to obtain the best oleogel in terms of texture and stability, which can then be used also to produce oil foams.
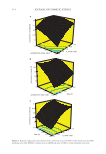
400 JOURNAL OF COSMETIC SCIENCE Graphical Abstract INTRODUCTION Oleogels are a class of soft materials that can entrap large volumes of liquid oils in self- assembled network (1). The presence of this network provides viscoelastic, or even gel- like properties. The formation of oleogels, which are systems containing mostly oil as the solvent, is a phenomena of interest for various applications ranging from food to cosmet- ics and pharmaceuticals to paints but also, more recently, for drug delivery and templat- ing structures (2). The addition of specific polymers to the oil phase leads to oleogelation (3). For example, triblock copolymers of the Kraton type are used in cosmetic industry (4). Ethylcellulose is described in the literature for food application (5). Low molecular weight gelators can also be used to gel oils and are often able to gel oils at already very low concentrations of 0.1–1 wt.%. For this type of oleogels, immobilized oils keep the fluidity of the bulk liquid at the molecular level as shown by NMR self-diffusion experiments (6). One of the best known low molecular gelators in cosmetic industry is 12-hydroxystearic acid (7–11). Recently, studies have investigated new oleogelator systems for industrial applications including waxes, ceramides, ethyl cellulose, proteins, and so on (12–15). Moreover, combinations of known oleogelators such as fatty acids and fatty alcohols have also received a renewed interest (16). These two fatty components are both low molecular gelators and they can be used for food, pharmaceutical or cosmetic applications (17). Fif- teen years ago, Gandolfo et al. were the first ones to show a synergistic effect for specific weight ratio (R) between fatty alcohol and fatty acid in oleogels (18). The authors showed a clear effect of R on the hardness of oleogels for the mixture of stearyl alcohol and stearic acid (18). Two optimums were found for oleogels based on sunflower oil: R = 7:3 and R = 3:7 (w/w). Recent studies demonstrated that the effect of R on textural properties of oleo- gel was because of the formation of small, platelet-shaped mixed crystals (19). An almost complete crystallization for these two R was observed. The parameters associated with a suitable spatial distribution of the crystals inside the oleogel led to an increase of hard- ness and stability of these oleogels (20). Therefore, the combination of stearyl alcohol and stearic acid is an easy way to obtain an oleogel more stable over time and temperature, and to improve as well as their mechanical properties. These two criteria are important for the commercial applications of these oleogels. For the system based on stearyl alcohol
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)