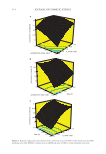
385 SKIN PERMEATION OF HAZARDOUS COMPOUNDS HS-GC-MS DETERMINATION Analysis of hazardous organic compounds in receptor solutions was performed using an Agilent 7697A head space (HS) injector, a 7890A GC, and a 53975C inert XL EI/CI MSD with triple-axis single quadrupole detector. Five milliliter receptor solution and 200 ng mL−1 of internal standard (toluene-d 8 ) were introduced in a 10-mL HS glass vial. HS vial was hermetically closed, heated at 60°C for 20 min, and HS was measured by GC-MS. Injector temperature was 250°C, employing 0.8 mL min−1 constant flow helium as carrier gas. Capillary column and GC oven temperature program were that previously described in TD-GC-MS analysis. Electron-impact ionization was performed at 70 eV and MS acquisitions using selected ion monitoring (SIM) mode. LC-MS-MS Nicotine determination in receptor solution was performed by LC-MS. An UHPLC-MS instrument model ACQUITY ® TQD, from Waters (Milford, MA), with a KINETEX C18 evo (50 x 2.1 mm, 1.7 µm) column, from Phenomenex (Torrance, CA). Mobile phase consisted of 50 mM ammonium acetate in water (A) and methanol (B). Gradient elution from 5% to 95% mobile phase B in 2 min was used with a flow rate of 0.4 mL min−1, a 5 µL injection volume, and 30°C column temperature. MS acquisitions were done using 3.5 kV capillary voltage, 120°C source temperature, 300°C desolvation temperature, and 690 L h−1 desolvation gas flow rate. Multiple reac- tion monitoring (MRM) conditions were adjusted for nicotine and nicotine-d 4 , being the transitions m/z 163 → 130 and 167 → 136, respectively selected. Table II Analytical Features, Including Selected Ions and Retention Time, of the Organic Compounds Determined in Cigarette Smoke by Active Sampling and Analyzed by TD-GC-MS and HS-GC-MS Analyte Ions (m/z) Retention time (min) Lineal range (ng) LODa (ng) LOQb (ng) R2 Benzene 77, 78 2.50 20–4,000 6 20 0.995 Toluene 91, 106 4.43 20–4,000 6 20 0.998 Chlorobenzene 112 7.63 200–4,000 50 170 0.978 Ethylbenzene 91, 106 8.57 100–4,000 30 100 0.997 m+p-xylene 91, 106 9.27 200–4,000 60 200 0.992 Styrene 116 10.62 500–4,000 150 500 0.984 o-xylene 104 10.65 200–4,000 50 170 0.994 p-Cymene 91, 117 13.40 350–4,000 100 330 0.985 Limonene 67, 93 13.47 200–4,000 60 200 0.999 Naphthalene 128 15.10 20–4,000 5 17 0.994 Nicotine 84, 133 16.30 200–4,000 50 170 0.989 Acenaphthylene 152 17.00 20–4,000 5 17 0.979 2-methylanthracene 192 18.32 100–4,000 25 83 0.996 1-methylphenanthrene 192 19.60 100–4,000 25 83 0.996 a Limit of detection b Limit of quantification
386 JOURNAL OF COSMETIC SCIENCE RESULTS AND DISCUSSION IDENTIFICATION OF TOBACCO SMOKE COMPOUNDS INSIDE THE SIMULATION CHAMBER Cigarette smoke is an aerosol consisting of small, gas-phase-suspended droplets with a complex chemical composition of over 8,700 identified constituents (27), but it has been estimated that the actual number may approach 100,000 (28). A vast amount of liter- ature has appeared since 1950 on tobacco-smoke constituents (29,30,31). Compounds from cigarette smoke can be classified as neutral gases, carbon and nitrogen oxides, amides, imides, lactames, carboxylic acids, lactones, esters, aldehydes, ketones, alcohols, phenols, amines, N-nitrosamines, N-heterocyclics, aliphatic hydrocarbons, monocyclic and polycyclic aromatic hydrocarbons, nitriles, anhydrides, carbohydrates, ethers, nitro compounds, and metals (32). The aim of this paper is not to provide a complete list of organic compounds from tobacco smoke but to demonstrate the usefulness of the simulation chamber to generate a pol- luted atmosphere that represents a situation close to the real one. In this sense, Table III provides a list of the potentially identified organic compounds after active sampling of the simulation chamber air during the simultaneous combustion of three cigarettes using the developed smoking machine. Identification has been done by TD-GC-MS after comparison of the obtained MS spectra with those of National Institute of Standards and Technology (NIST, Gaithersburg, MA) Mass Spectral Library, containing more than 300,000 MS spectra of organic compounds. Moreover, when possible, GC retention time Table III Retention Time and Selected Ions of Identified Analytes from Cigarette Smoke Inside the Simulation Chamber t R (min) m/z Potential analytes 1.61 43 Isoprene 1.95 55, 56 1-nitropentane 2.02 41, 43 Trans cyclopent-1-en-3,5-diol 2-, 3-methylfuran 2.30 77, 78 1,4-cyclohexadien 2.52 77, 78 Benzene 2.82 43, 55 2-methyl-4-pentenal Tetrahidro-4-methyl-3-methylenfuran 2.99 43, 79 5-methyl-1-hexin 4-isopropylcyclohexanol 3.08 95, 98 2,5-dimethylfuran 3.40 65, 66 Phenol 3.76 91 1-(1,3-butadienyl)-2-vinylcyclobutane [(cyclohex-1-en-3-yl)methyl]benzene 3.99 79 Pyridine 4.21 68 5,5’-oxibis[(E)-1,3-pentadiene] t-butyl acetylene 1S-(-)-N-(cyclopent-2-en-1-yl)hydroxylamine 4.43 91, 92 Toluene 4.61 77 5,5-dimethylcyclopentadiene 1-methylcyclohexa-2,4-diene (Continued)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)