411 THE EFFECT OF VEGETABLE OIL COMPOSITION result, since height peaks were identified in the WAXS regime. Five of these peaks corresponded to the ones determined previously for R = 8:2, 7:3, and 5:5 giving the same d-spacings (4.6, 4.5, 4.0, 3.8. and 3.6 Å). Three additional peaks were observed with d-spacings corresponding exactly to the ones obtained for the oleogels containing pure BA crystals (4.3, 4.15, and 3.7 Å). All the d-spacings are presented in Table II. The scattering intensity of the peak corresponding to the pure BA crystals increased from R = 3:7 to R = 2:8, whereas the scattering intensity of the peak corresponding to the mixed crystals decreased. The quantity of mixed crystals decreased in favor of pure BA crystals by increasing R. The same effect of R was observed in terms of crystalline particles structure, regardless of the oil used to produce them. All the oleogels had the same crystalline structure as a function of R, which explained why all the oleogels exhibited the same thermal behav- ior by DSC. Mixed crystals between BO and BA were present for three ratios: R = 8:2, R = 7:3, and R = 5:5. The smallest crystalline particles were observed for R = 8:2 and R = 7:3, which correspond to molar ratios of around 2.4 and 4.2. The smallest crystals observed for R = 8:2 and R = 7:3 in the BO:BA system in comparison to the other ratios could come from a decrease in interfacial tension for these specific ratios (18). Indeed, in the literature, the same effect was observed by Gandolfo et al. for oleogels based on the mixed systems stearyl alcohol and stearic acid (18). Monolayers based on stearyl alcohol and stearic acid exhibited a minimum area per molecule leading to a decrease in inter- facial tension for molar ratio equal to 3:1 (45). Therefore, Gandolfo et al. supposed that the nucleation rate linked to the interfacial energy increased for specific ratio in oleogels (18,46). This decrease of the interfacial energy could result into a decrease in the aver- age crystal size (18,46). We suppose that in BO/BA system the same behavior could also occur for a molar ratio of 3:1 corresponding to an optimal weight ratio comprised between the weight ratio R = 8:2 and R = 7:3. We proposed a schematic phase diagram to summarize our DSC and SAXS/WAXS results (Figure 5). For R =8:2, R = 7:3, and R = 5:5, mixed crystals of BO and BA were observed (one crystalline phase). For R = 3:7 and R = 2:8 the mixed crystals were present with pure BA crystals: two crystalline phases. As shown by DSC, the mixed crystals melt before the pure BA crystals for R = 3:7 and R = 2:8. This proposed phase diagram was in accordance with the phase diagram for the binary stearic acid/stearyl alcohol system in oil proposed by Bot and Flöter (16). OIL PROPERTIES AND LINK WITH OLEOGEL PROPERTIES From our multiscale approach, we did not observe an effect of the vegetable oil on the oleogel structural properties. The evolution of the microstructure observed by optical microscopy as function of R remained the same whatever the vegetable oils. In the same way, we determined by combining DSC and SAXS/WAXS experiments that the crystal- line structure evolution as a function of R was the same for all the oils studied. We only observed an effect of oil on the oleogel physical properties, oleogels hardness, and oil loss. We compared the results described in this study with the previous ones obtained for the same BO/BA oleogelator system in the same conditions in sunflower oil, for which the optimal R was 7:3 (27). The oleogels were classified into two groups based on the optimal R obtained in terms of highest hardness and lowest oil loss during centrifugation process. The first group composed of sunflower, apricot, and rapeseed oils exhibited an optimal
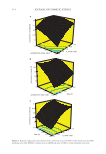
412 JOURNAL OF COSMETIC SCIENCE ratio at R = 7:3. The second group composed of olive and camelina oils exhibited an optimal ratio at 8:2. To understand what is the link between the vegetable oils inside the two groups, we deter- mined three oil properties at 25°C: surface tension, viscosity, and density (Table III). Sun- flower, olive, apricot, and camelina oils had surface tension around 33 ± 0.7 mN·m−1. Only rapeseed oil exhibited a slightly higher surface tension around 35.4 mN·m−1 and was statis- tically different from the other vegetable oils (Table III). Therefore, in terms of the surface tension, a link was not found between the two groups found previously. For the viscosity, sunflower oil had the highest viscosity around 70.2 mPa.s. Camelina oil had the lowest one around 52.7 mPa.s. Olive and apricot oil had close values around 62.5 and 60.8 mPa.s, respectively. Rapeseed oil had a value around 56.1 mPa.s. Again, we found no link between the two groups obtained previously and the viscosity of the different oils. Moreover, all the vegetable oils had the same density from statistical analysis around 0.9 showing that the density was not the key parameter giving rise to the different oleogel properties as a function of the oil. In contrary to previous study on oleogels based on γ-oryzanol and β-sitosterol, which have shown that the viscosity of the oil phase affected the final gel strength of the oleogels, no link was observed in the case of the BO/BA system (35). A key parameter described in the literature to affect the oleogel properties is the percent- age of unsaturated fatty acids in oils (33). We compared the fatty acid chain unsaturation composition for all vegetable oils with oleogel properties (Table I). We observed that for the first group formed by the sunflower, apricot and rapeseed oils, there was no link between the oil compositions in terms of unsaturation of fatty acid. For example, sun- flower oils contained 27.2% of oleic acid and 58,7% of linoleic acid, whereas apricot oil contained 60% of oleic acid and 29.1% of linoleic acid. In the same way, for sunflower oil with optimal R = 7:3, the total percentage of unsaturation in oils was around 86.9 wt.% and it was close to 86 wt.% for camelina oil with an optimal ratio for R = 8:2. Another parameter already mentioned in the literature which could play a role on the oleogel properties was the fatty acid chain length composition of the vegetable oil (39). We compared the fatty acid chain length composition of vegetable oils with the oleogel properties (Table I). We observed that for the first group formed by the sunflower, apricot, Figure 5. Schematic phase diagram for BO/BA oleogelator system in oil. One phase region is indicated by 1φ, and two phases region is indicated by 2φ. S corresponds to solid and L to liquid. Platelets crystals are drawn: pure BO in red, mixed BO/BA crystals in red and blue dashed, and pure BA in blue.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)