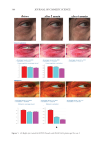
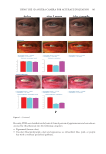
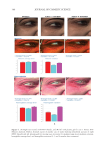
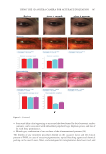
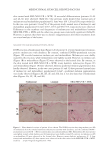
358 JOURNAL OF COSMETIC SCIENCE As our shampoos S2, S3, and S4 had much more silicone deposition, it would not affect our discussion. There was almost no deposition for S1 on natural hair. We observed a very small quantity of silicone deposition for natural hair compared with damaged hair for all shampoo samples. Natural hair treated with shampoos S2 (0.21% amodimethicone) and S3 (0.42% amodimethicone) showed more silicone deposition compared to shampoo S1 (base shampoo) hair treated with S4 (0.18% silicone quaternium-18) showed much more silicone deposition than S1 (base shampoo), S2, and S3 shampoos. Between S2 S1 S2 S3 S4 Figure 10. Digital microscope observation of Asian damaged hair after 28 washes by each shampoo. S1: base shampoo without silicone S2: shampoo with 0.21% amodimethicone S3: shampoo with 0.42% amodimethicone S4: shampoo with 0.18% silicone quaternium-18. 0.00 100.00 200.00 300.00 400.00 500.00 600.00 700.00 S1 S2 S3 S4 Natural hair Damaged hair Figure 11. Silicone deposition results on Asian natural hair and damaged hair washed by each shampoo. Means +/− SD. S1: base shampoo without silicone S2: shampoo with 0.21% amodimethicone S3: shampoo with 0.42% amodimethicone S4: shampoo with 0.18% silicone quaternium-18. The baseline was extracted for all deposition data. Silicone Deposition (μg/g ha)ir
359 Silicone Reduce Combing Force, Flyaway, Damage in Shampoo and S3, the difference was not so large. For damaged hair, the silicone deposition was enhanced much more for shampoos S2, S3, and S4 compared to S1. Hair treated with shampoo S4 showed the largest silicone deposition, and hair treated with shampoo S3 showed more silicone deposition than S2 the increased silicone deposition quantity was larger than that of natural hair. More silicone deposition was observed on damaged hair than on natural hair for all the shampoo samples. These results demonstrated our second hypothesis: the amine group or cationic group in silicone molecules can aid in effective silicone deposition on hair the cationic group can aid in even more silicone deposition on hair. As we already explained, amodimethicone, because of its hydrophilic moiety of NH 2 group and its cationic charge, is relatively easy to deposit on damaged hair. We also observed the increase of silicone deposition when we increased the quantity of amodimethicone, and although in the formulation S3 contained twice the quantity of amodimethicone compared to S2, the silicone deposition was not double for damaged hair. In addition, since silicone quaternium-18 is a cationic silicone, we supposed it may also form coacervate with the surfactant so it is easier to deposit on damaged hair or the damaged part of natural hair the deposition efficacy was higher than with amodimethicone, even at less concentration at formulation (0.18%). Our silicone deposition quantity was comparable with the reference (13). Haake et al. also used ICP-OES to measure the silicone deposition quantity, although we used a digestion hair sample to measure the silicone deposition quantity. Haake et al. used an extraction method to extract the silicone adsorbed from hair with a mixture of o-xylene and isopropanol. Their silicone deposition quantity was about 400–650 µg/g when they tested a two-in-one shampoo (contained dimethicone) purchased from a German market with a Caucasian hair swatch, and tested another two-in-one shampoo (contained dimethiconol) purchased from a Thailand market with a Japanese hair swatch, which is very similar to our data of S2 and S3 on damaged hair and much bigger than our deposition data on natural hair. The silicone deposition is due in part to the particle size and the quantity used in the shampoo formulation. It is unknown what particle size and quantity of silicone was used in the two-in-one shampoos purchased from German and Thailand markets. It is clear that our amodimethicone microemulsion and silicone quaternium-18 microemulsion will not deposit to a greater extent than dimethicone and dimethiconol on natural hair and damaged hair. Dussaud et al. (5) used streaming-potential measurement and determined the deposition of the amino-polyether-silicone block copolymer ([AB]n copolymer), which was found to be about 800 µg/g on undamaged hair when they dipped the hair for 1 hour in the treatment when the [AB]n copolymer concentration was 0.5mg/g (0.05%), pH4 and ionic strength 0.001 M. Although Dussaud et al. used a different hair treatment method and different measurement for deposition, their data were still referable. Our deposition data of silicone quaternium-18 on damaged hair were relatively lower than the results of Dussaud et al.’s data, and much lower on natural hair. Our deposition data on damaged hair were not high and were also verified by a sensory test. A greasy feel, typically associated with large deposition of silicones, was not perceived on damaged hair after 28 shampoo washes. This also demonstrated our third hypothesis: controlling the amine and cationic group quantities at a certain level, and balancing the hydrophobic and hydrophilic moiety of the silicone molecule could avoid heavy deposition. The silicone deposition results had very good correlation with combing test results, flyaway control results, hair breakage test results, tensile strength results, and digital microscope observation results. It indicated that the deposition of amodimethicone and silicone quaternium-18 on hair has contributed to the combing force reduction, flyaway control, less hair breakage, good tensile strength, and damaged hair reparation.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)