DIFFUSION PROCESSES IN HUMAN HAIR 601 the requisite period, the holder and tubes are removed, and the dye remaining in the capillary tubes is estimated. The diffusion constant is calculated from Dt _• (1- Cav'• 2 (11) h 2 4 C O ,/ where Cav is the average concentration within the tube at the end of the experiment, C O is the concentration at the beginning of the experiment, and h is the height of the capillary tube, which has been shown to hold provided Dt -- 0.2. Diffusion constant in hair White, untreated hair, was either pre-treated or added dry to the dye bath in the ratio of 1 g of hair to 100 ml of bath. The bath, which was not agitated, was maintained at a constant temperature. The hair was removed at the appropriate intervals and rinsed quickly in cold water. The dye taken up by the hair was extracted with pyridine-water or with dimethyl formamide. RESULTS AND DISCUSSION The technique for determination of diffusion constant in water was checked using paminobenzoic acid. The determined value was 6.57 X 10 -6 cm•/sec, which is somewhat lower than the published value (11) of 8.42 X 10 -6 cm'/sec. Experimental errors will, in general, tend to give higher values rather than lower. The method was assumed to be working satisfactorily. The limited available data on the diffusion constant at 25 ø C in water and in hair, of a range of dyes of varying size, are summarised in Table II. Table II Diffusion constants in hair and in water Diffusant Dwater X 10 6 Dhair X 10 •2 Dhair (cm2/sec) (cm•/sec) Dwate--• X 10 6 (•) A 4.0 12-0 3-0 5.3 B 3.3 9-0 2.6 6-4 C 12-0 260 20.8 1 '8 D 8.6 480 55.9 2.6 E 10.0 316 31.6 2.1 The determined values for the radii seem, at first sight, rather small for dye molecules. The radius is, however, that of a sphere of equivalent volume, and although the dyes may be relatively long, the width and the thickness are not very great. Thus, even with the compact molecule, paminobenzoic acid, the approximate dimensions are 9 X 6 X 2 A, which
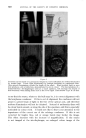
602 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS gives an approximate radius for the equivalent sphere of 3.0 A. This is in fair agreement with the determined value of 3-7 A. Valuesof (D•)lare plottedagainstrdinFig.lineofbestThe2. is drawn through these points. The intercept of 7.4 A indicates that the holes are 14.8 2k across. This is intermediate between the values which WiNmann found and those predicted by Speakman. Considerably more data are required, however, to attach any confidence to the result. 10. o z 4 6 $ • (4) Figure 2 The effect of diffusant radius on the relative diffusion constant. Diffusion coefficients have been determined for one dye (C) at different temperatures. At 25 ø C, the value is 257 X 10-" cm2/sec, but at 60 ø C it is 4,422 x 10-" cm=/sec. This corresponds to an activation energy of 16,450 cal/mole/ø C. This is not exceptionally high for a fibre, and is a reflection of the large change in the viscosity of the water within the fibre as the temperature rises. This is not uncommon. For instance, if we calculate the theoretical activation energies of diffusion in a sucrose solution from viscosity data, we have the results in Table III. Table III Activation energies of diffusion in sucrose solutions Sucrose 0 20 25 70 75 cal/mole 4,735 5,442 5,689 14,948 18,588
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)