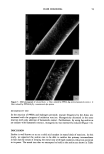
20 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS • 125 0 100- • 75- •'• 252 el" o.oool I I I I I SDS SOLUTION 1 ER JR 400 , , ,If , O.OO1 O.O1 O.I 1 .O IO.O SDS CONCENTRATION % Figure 1. Relative viscosity of 1% Polyquaternium-10 (JR400), SDS mixtures as a function of SDS con- centration. latter property can be especially pronounced if the polymer has a "stiff" backbone, as in cellulosics (see Figure 1). Much smaller effects were obtained with a typical "vinyl" polycation, viz., Polyquaternium 5 (6). Development of high viscosity in the former case is taken to be evidence of the forma- tion of a structured network through alkyl chain association of surfactant molecules bound to cellulose chains. It seemed that these effects could be promoted, with the development of gelling structures, if the molecular weight of the cationic cellulosic polymer were to be increased. This paper reports such an investigation using the highest available molecular weight grade Polyquaternium 10, viz., UCARE Polymer JR30M, in combination with various anionic surfactants. BACKGROUND: WHAT IS A GEL? The current picture of a gel is of a state in which a structuring additive, such as a finely divided solid or a dissolved polymer, forms a crosslinked network, that is able to "hold up" or "solidify" a relatively large amount of entrained solvent. The network that forms endows the largely liquid composition with the classical property normally associated with the gel state, i.e., viscoelasticity. Aesthetic and practical considerations often dictate a preference for polymeric rather than solid particulate gelling agents. For fur- ther information on gels, several review articles are available [see, for example, reference (7)]. Traditionally, the foods and pharmaceutical areas have had an active interest in gel technology the cosmetic industry, while also practicing this technology, has done so to a lesser degree. Current patent literature, however, indicates an upsurge of interest in gels in the cosmetics field. The objective of this work was to establish whether or not a combination of a high-molecular-weight cationic cellulosic polymer at low concentra- tion (--1%) and selected anionic surfactants could form a range of gelling compositions and, if so, to characterize the systems rheologically.
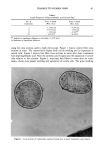
POLYMER/SURFACTANT GELLING STRUCTURES 21 RHEOLOGICAL CHARACTERIZATION OF GELS Since gels are viscoelastic materials, i.e., exhibit both liquid and solid characteristics, conventional rheological methods, viz., viscosity determinations, are not the most useful or informative way to study them. Anomalous behavior can be encountered, such as "climbing" of the specimen up the shaft of the rotor, in a typical rotational visco- metric determination. To obtain more complete and reliable information, viscoelastic- ity is better studied by oscillatory measurements. Here a sine wave strain, instead of a constant rotational strain, is applied to one of the elements in a typical measuring pair, e.g., concentric cylinders, and the stress and phase angle are measured on the second element. This allows determination, as seen below, of both the storage (elastic) mod- ulus and the loss modulus (representing viscous flow). For a viscoelastic material, the phase angle will be between 0 ø and 90 ø. For a purely elastic material, the generated stress is always in phase with the strain, i.e., 8 = 0 ø. For a purely viscous material, there is a 90 ø phase difference between the two (see Figure 2). The magnitude of 8 establishes the relative contribution of the elastic and viscous com- ponents according to the following equations: Complex modulus G* = 'ro/'y o = G'+ iG" Elastic modulus G' = G* cos8 Loss modulus G" = G* sin8 Dynamic viscosity qq = G'%o where I•o and •o are the stress and strain amplitudes, •o is the oscillation frequency in radians/sec, and i is the imaginary constant, :k/-•. t, (a= Figure 2. Phase relationship of stress (% and strain 0/) for a viscoelastic material.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)