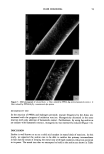
30 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 1% JR 30M / VARIOUS SURFACTANTS MODULI at 0.2 Hz 1 ooo ! U__quid Like • O.a%ES2 / O.i••DBS / 0.2•AOT Gel Like 10 10 100 1000 G' (Pa) Figure 12. Composite plot o[ citric modulus (G') versus loss modulus (G") showing the points o[ max- Jmum gc[ strength [or the •J•crcnt sur•ac•ants Jn combination with l• Po]yquatcrnJum-10 OR30M). complete clarity of the latter. It is of interest that when the experiment was done in reverse, i.e., small amounts of JR30M were added to 1% CMC solution, no gels were formed instead, mobile, turbid suspensions were obtained. "NON-RHEOLOGICAL" TEST RESULTS VISUAL INSPECTION Simple tilting of the containing vessel and examination by eye yielded initial informa- tion concerning the presence of gels, and the approximate surfactant/polymer ratio for optimal gelling was easy to establish by this test. At 1% polymer, and the optimal ratio of anionic surfactant, the compositions were largely immobile and very resilient for example, the containing vessel could be up-ended and the gel would remain attached to the (now) upper surface of the vessel with no evident flow for a week or more. Likewise, in the simple cylinder shake test employing 0.2% polymer solutions, air bubbles re- mained entrapped and in suspension for several days if the system were near or at the optimal ratio for gelling. By contrast, the entrained bubbles disappeared from a 0.2% polymer control system after a few hours. ELECTRON MICROSCOPY Dramatic differences in the appearance of films formed by freezing and drying non-
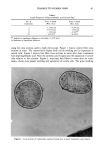
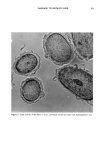
POLYMER/SURFACTANT GELLING STRUCTURES 31 MODULI at 0.2 Hz 1% Polymer / Optimal [SDS] l0 a , • Liquid Like 10 2 - - •, HR• JR30M _ • LR30M _ 1 ol ••400• - •R/• Gel Like 10 ø ' , • 10 ø 101 10 •- 10 3 O'(Pa) Figure 13. Composite plot of elastic modulus, G', and loss modulus, G", for different cationic cellulosic polymers (1%) with SDS at concentration of maximum gel strength. gelling and gelling compositions (1% JR30M and 1% JR30M/0.1% SDS, respectively) were found. Whereas the films from the former tended to be flat and featureless, the latter generated a honeycombed network shown at two magnifications in Figure 14. While these electron micrographs are highly suggestive that this structure may in fact represent the actual elastic network present in the gel, it must be emphasized that it may be an artefact connected with the high consistency of the original gel and of its effect on the drying process itself. METHODS TO LIQUEFY THE GELS Situations may arise in which it is desired to liquefy a preformed gel. The most straightforward way would seem to be to add either one of the two components so as to move the ratio away from the maximum gelling value. Each of these approaches, on the other hand, may be disadvantageous: the high-molecular-weight polymer may be diffi- cult to post-incorporate, whereas addition of extra surfactant may take the system into the precipitation zone, which may be objectionable. Other approaches are: a) Adding a compatible cationic mrfactant that will compete with the polycation for the anionic mrfactant and so weaken the polymer/mrfactant network structure. It was found that (post) adding an equal quantity of an alkylated methyl glucoside quaternary to polymer usually led to pronounced thinning of the gel. b) Adding salt to reduce the electrostatic interaction between the polycation and the anionic sur- factant. This approach turned out to be of limited effectiveness.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)