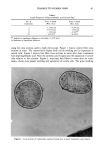
28 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS % JR 30M Optimal [SDS] 1 ooo 1 oo lO 1 o.1 o.1 U_quid Like 0.2% (}.el Like , , 1 1 o 1 oo 1 ooo G'(Pa) Figure 9. Comparison of G' and G" for mixtures of Polyquatemium-10 (JR30M) and SDS, at maximum gel strength concentration, as a function of polymer concentration. 1% JR 30M at 0.2 Hz 400 100 300 200 lOO 90 0.1 0.2 80 70 60 • 50 ,•. -4-0 • - 30 - 20 -10 o o o.o 0.3 % SDS Figure 10. Comparison of elastic modulus, G', and phase angle delta, •, for mixtures of Polyquaternium- 10 0R30M) (1%) with SDS as a function of SDS concentration.
POLYMER/SURFACTANT GELLING STRUCTURES 29 1 To JR 30M at 0.2 Hz 400 300 --- ' t 200 o • • o.o o.1 0.2 % SDDBS Figure 1 la. lOO 9O 8O 70 6o ,:% 50 • ..o 40 '• 3O 2O 10 0 0.3 1% JR 30M at 0.2 Hz 400 300 2OO 100 C• I I 0.0 0.1 0.2 % AOT Figure 1 lb. lOO 8o 7o• 50 40 c• o 0.3 400 1% JR 30M at 0.2 Hz 300 200 100 0 • o.o o.1 0.2 % ES2 Figure 1 lc. 1 O0 400 I 8O 30O 70 5 - 60 • 50 • • 200 ..e b 40 • lOO 1% JR 30M at 0.2 Hz lOO lO o 0 0.3 0.0 0.1 0.2 %ES3 Figure 1 ld. 90 80 7O 60 • 50 • 4o • 50 2O 10 0 0.5 Figure 11. Comparison of elastic modulus, G', and phase angle, 8, for 1% Polyquaternium-10 (JR30M), with various added surfactants as a function of surfactant concentration: a) DDBS. b) AOT. c) ES-2. d) ES-3. EFFECT OF POLYMER Figure 13 shows first that, in combination with SDS at the optimal ratio for gelling, the two highest molecular weight cationic cellulosic polymers yield points lying in the "elastic" field whereas the lower molecular weight homologs yield points in the viscous field second, there is a clear trend of the points for higher CS polymer homologs to lie in the higher consistency regions of the G", G' graph, i.e., towards the upper right of the plotting field. The latter effect arises from a higher level of cross-linking achieved via alkyl chain association of surfactant molecules bound to the cationic sites of the polymer. Addition of the high-molecular-weight polyanion, CMC, at the 0.1 or 0.2% level to 1% JR30M solutions also resulted in the formation of gels. These gels, however, were not as strong as those made with the dodecyl sulfate anion and furthermore lacked the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)