DYES AND SURFACTANTS FOR HAIR 5 were removed and diluted with 1.5 ml EtOH for UV measurements. The amounts of adsorbed surfactant were calculated from the absorbance at 261.5 nm. The depen- dencies of absorbance versus concentration for all investigated surfactants were found to follow Beer's law. The extraction was carried out on 0.5 g of hair fibers obtained after the 24-hour adsorp- tion experiment, with a 5-ml mixture of 75% ethanol/25% water at room temperature. The amount of extracted surfactant was evaluated by means of UV spectroscopy. UV-Vis spectroscopy. The absorption spectra were monitored by using a Perkin-Elmer 553 Fast Scan UV/Vis spectrophotometer. Treatments of hair tresses with surfactants. Treatments with reactive surfactants were per- formed on both intact and reduced hair. Intact hair tresses (2 g each) were exposed to 1% solutions of surfactants (liquor/hair ratio 5:1) at a specified pH for 30 min at room temperature, followed by rinsing, combing measurements, and shampooings. Reduction was performed by treating hair tresses with 6% ammonium thioglycolate (adjusted to pH 9, liquor/hair ratio 12.5:1) for 10 min at room temperature and rinsed with tap water for one minute. Then the hair was exposed to 10 ml of 1% solution of the corresponding surfactant at a specified pH for 5 or 30 min at room temperature, followed by neutralization with 10 ml of 3% H202 for 8 min, and then rinsing. All shampooings entailed lathering each tress with approximately 0.5 g anionic surfac- tant-based shampoo for 30 sec, followed by rinsing with warm tap water for 30 seconds. Treatments of hair tresses with dyes. Blended gray hair swatches were treated with 11-, 24-, and 55-mm dye solutions at a liquor/hair ratio of 2:1 and at pH 5 and 10 for 30 minutes. The swatches were then rinsed with warm tap water, blow-dried, and ana- lyzed on a Hunter Colorimeter. The Hunter tristimulus values were remeasured after shampooing (performed as described above) each of the dye-treated swatches six times with an anionic surfactant-based shampoo. Dyes were also applied to hair (a) after reduction with ammonium thioglycolate, before oxidation with H202 (b) during the reduction, in combination with ammonium thio- glycolate and (c) during the oxidation, in combination with H202, following the re- duction with TGA. The evaluation of the washfastness of the treatments was performed as described above. Combing work measurements. The conditioning effect, and its durability after multiple shampooings, was evaluated based on wet combing work measurements performed ac- cording to the protocol described by Garcia et al. (17). An Instron Model 1101 tensile tester and 2 g (6.5-inch long) hair tresses were used in all experiments. Wet hair was first combed to remove entanglements, dipped three times in water to introduce entan- glements in a controlled fashion, and then mounted in the Instron with hair evenly distributed across a 1-inch length of comb. The combing force was continuously re- corded as the tress was combed at a crosshead speed of 10 cm/min, and its integrated value over the length of the tress was calculated as the combing work. The data reported in this paper are the average values of combing work obtained for each of two tresses. The overall error of combing measurements on intact and reduced hair is estimated to be about 25% and 50%, respectively. Evaluation of color difj•rences. The Hunter tristimulus L,a,b values were measured by the use of a Hunter Colorimeter Model D25-9. The most informative parameter was the b
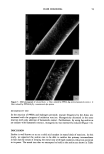
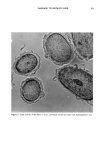
6 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS value, which measures yellowness (positive values), gray (zero), and blueness (negative values). The reported data are the average obtained from the reflectance, measured at several positions on a hair swatch. Microscopy. Cross sections (30-1xm thick) of dye-treated hair were cut from collodion- mounted fibers by the use of a microtome. They were photographed with a Pentax camera attached to a Leitz optical microscope at 200 and 312 magnification. RESULTS SORPTION AND EXTRACTION OF SURFACTANTS Quantitative sorption and extraction studies were performed by the use of surfactants 11-13, which could be quantified in solutions by means of UV spectrophotometry. Figures 1 and 2 present the time dependencies for sorption of these surfactants from aqueous solutions at pH 5.3 and 10.7 on untreated hair. They indicate that the hy- droxyl- and bromide-containing surfactants readily deposit on hair and, after 24 hours, the solutions are almost completely depleted. The equlibrium ad(b)sorption was found to be about 30-35 mg/g, which is considerably more than the amount corresponding to a mono- or bilayer coverage of the fiber surface (below 1 mg/g). Thus the deposition is probably due to both adsorption and absorption of the surfactants into the cuticles or 40 35- o 20- o E 10- _ ,/ /+ ,/ + 2 4 pH 5.3 0 6 I• 10 1'2 1'4 1'6 1 2'0 2'2 24 Time (hours) alcohol (11) + bromide (12) x isothiuronium](13) Figure 1. Amount of surfactant ab(d)sorbed as a function of time for unmodified hair at pH 5.3.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)