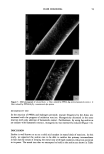
DYES AND SURFACTANTS FOR HAIR 9 Table III Results of Wet Combing Work on Intact Hair Wet combing work (g/cm) pH of the After After After Treatment (30 min) Treatment treatment 4 shampoos 8 shampoos Control 5.3 952 3006 2941 10.7 1114 1855 3270 Stea•lkonium chloride 5.3 715 1078 1704 10.7 888 640 1504 Compound 5 5.3 697 962 1804 10.7 1282 660 1573 Compound 6 5.3 688 986 1878 10.7 1222 1068 1542 Compound 7 5.3 719 594 1108 10.7 1004 340 708 Compound 8 5.3 722 1162 1872 10.7 1091 666 1918 Compound 9 5.3 785 846 1744 10.7 1038 553 2384 Compound 10 5.3 552 630 1074 10.7 303 207 582 the treatment at pH 10.7 is higher than that for the tresses subjected to four sham- pooings. This might be caused by the enhanced absorption of the surfactant at this pH, which may lead to less adsorption, and consequently, less surface modification. Subse- quent shampooing of the hair results in a decrease of combing work values, probably because of diffusion to the surface and formation of surface hemimicelles of the absorbed surfactant. This and alternative mechanisms are discussed further in the text of this paper. An additional four shampooings produce an increase in combing forces, due to removal of the quats from the surface. Similar combing characteristics are displayed by the hydroxyl- and bromide-containing cationic surfactants. In both cases, treatments at pH 10.7 resulted in relatively high combing work, which was subsequently reduced by four shampooings. Also, no qualitative difference in performance was observed for sur- factants with one and two hydroxyl or bromide groups. Both isothiuronium-containing quats were considerably more effective in reducing the combing work than the nonreactive surfactants, especially when applied at high pH. The conditioning effect was also more durable, and clearly evident after eight sham- pooings. The data presented in Table III also indicate that the surfactant containing two isothiuronium groups (compound 10) produces a more significant reduction in combing forces than its monoisothiuronium analog. The combing work measurements obtained for the treatments performed on reduced hair are presented in Table IV. According to the ad(b)sorption data, under these condi- tions the cationic surfactants diffuse into hair fibers at a high rate. Consequently, signif- icant and durable improvements in combing were observed for stearalkonium chloride and the hydroxyl-containing surfactants. The reduced hair treated with bromide-con-
10 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table IV Results of Wet Combing Work on Reduced Hair Wet combing work (g/cm) pH of the After After After Treatment (30 min) Treatment treatment 4 shampoos 8 shampoos Control 5.3 1515 3470 3846 10.7 1005 3625 4262 Stearalkonium chloride 5.3 1066 622 973 10.7 454 664 991 Compound 5 5.3 1521 794 1650 10.7 1044 642 1245 Compound 6 5.3 4036 1155 1538 10.7 4524 751 1474 Compound 7 5.3 2360 975 1645 10.7 1804 1587 1944 Compound 8 5.3 1354 555 1281 10.7 904 440 1210 Compound 9 5.3 3355 1165 2781 10.7 3911 2315 3176 Compound 10 5.3 264 523 616 10.7 278 904 1502 taining quats gave very high values of combing work, which were consequently de- creased after four shampooings. The greatest reduction in combing forces, durable through eight shampooings, was observed for the treatments with the diisothiuronium surfactant at pH 5.3. ABSORPTION AND SUBSTANTIVITY OF DYES The absorption of the control and isothiuronium-containing dyes was studied on both untreated and reduced hair. Substantivity of the dyeouts was evaluated by comparing the difference in Hunter b-values of the hair following dyeing and after six sham- pooings. Table V presents the results obtained for the treatments of blended gray hair performed utilizing various solvent systems at pH 5 (unhydrolyzed isothiuronium group) and 10 (partially or completely hydrolyzed isothiuronium group). The data indicate that 4 is considerably more washfast than 2 when applied from acidic solution, and only slightly more durable when applied from a solution at pH 10. Treatments with completely hydrolyzed 4, applied from a 60% EtOH-40% water solution at pH 10, were less substantive than the control dyeouts with 2. This seems to indicate that the expected disulfide exchange reaction between the hydrolyzed isothiuronium moiety of 4 and the keratin protein is not a significant factor in increasing substantivity of the dye. On the other hand, the cationic character of unhydrolyzed 4 may contribute to its observed higher substantivity to hair as compared to neutral 2. A relative increase in substantivity of the isothiuronium derivative was achieved by
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)