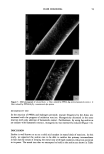
46 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The oxidative hair colorants consist of primary intermediates, oxidants, and couplers. Uncolored primary intermediates are oxidized by oxidants and produce the desired color in situ in the hair. In most cases hydrogen peroxide is employed as the oxidant. How- ever, it has been reported that the hydrogen peroxide reacts with the hair keratin and results in damage to the hair fiber (1-3). It is also well known that a strong peroxide solution will cause dermatitis When it comes in contact with the skin. In addition, B. N. Ames reported in 1975 that the oxidative products of p-phenylene diamine (PPDA), which is most generally used as a primary intermediate by hydrogen peroxide, resulted in mutagenicity (4). Therefore, much investigation has been carried out in the hope of finding a better and safer hair colorant (5). On the other hand, phenolic compounds were generally used as a coupler in oxidative dyes. Not only do they produce desirable color modifications, but they can also serve as color stabilizers. Tannin, which is contained in many kinds of plants, is expected to be a coupler because of its phenolic structures. In our previous study, it was found that hamamelis extract containing hamamelitannin (Figure 1) accelerated a polymerization of melanin. We expected that the hamamelis extract would accelerate the polymeriza- tion of oxidized PPDA and that the hamamelitannin in it would play a role as coupler. In this paper, we report the application of silver-zeolite as an oxidative catalyst and the use of hamamelis extract for the development of safety hair colorant. MATERIALS AND METHODS HAIR TRESSES Intact human gray hair tresses were used, purchased from Okamoto-shokai, Osaka, Japan. Approximately a gram of hair was used for each tress. The hair tress was washed in methanol, rinsed well with water, and air dried before use. ZEOLITE CONTAINING METAL IONS Metal-zeolites were obtained from Sinanen-New Ceramic Co. Ltd., Nagoya, Japan. Four kinds of metal zeolite were examined for hair coloring, Cu(II)-, Fe(III)-, Ag-(I)-, and Zn(II)-zeolite. There are some kinds of zeolite that differ from one another in their pore sizes. We obtained three types of zeolite whose pore sizes are 0.4 nm, 0.7 nm, and 1.3 nm. In addition, metal contents in zeolite may be changed. In the case of Cu-, Fe-, and Zn-zeolite, we used the zeolites containing 2.5 wt% of metal in 0.4-nm pores. In HO II o• •o• •H HO C-- , xOH Figure 1. Structure of hamamelitannin.
HAIR COLORING 47 the case of Ag-zeolite, we used seven kinds, which were 2.5, 5, 10, 15, and 20 wt% of silver in 0.4-nm pores and 2.5 wt% of silver in 0.7- and 1.3-nm pores. PLANT EXTRACT Hamamelis extract obtained from Maruzen-Seiyaku Co. Ltd. was used as the plant extract containing tannin. It was the extract with 1,3-butylene glycol. REAGENTS Other reagents used in this study were obtained from Nakarai Tesk, Kyoto, Japan. CONFIRMATION OF OXIDATIVE ABILITY OF METAL ZEOLITES PPDA was dissolved in Carmody buffer (Table I) at pH 9.6 in a concentration of 0.05%. 2.0 ml of PPDA solution, in which 0.02 g of metal-zeolite was suspended, was incubated at 25øC for ! min. After removing the zeolite by filtration, oxidation ability was judged with the naked eye in terms of the coloring of the tiltrate. HAIR COLORING Hair coloring was carried out in two steps. First the hair tresses were immersed in the solution containing plant extract. Then they were treated with PPDA solution in which Ag-zeolite was suspended. Both steps were carried out at 25øC. After coloring, the dyed tresses were rinsed under running tap water and air dried. PPDA was dissolved in a solution of about 1.5% ammonia and 5% isopropanol. COLOR EVALUATION OF HAll{ Color values and spectral reflectance data were obtained using the Spectrophotometric Color Meter CMC1200 (Murakami Color Research Laboratory). Three measurements were obtained per each tress and averaged. Results are expressed in the Hunter L, a, b color scale, where L is a measure of lightness and varies from 100 for perfect white to 0 for black. The coloring effect was evaluated with the L values only after coloring because of the color homogeneities in the gray hair tresses before coloring. Table I Carmody Buffer Composition Composition Conc. (M) A solution Boric acid 0.20 Citric acid 0.05 B solution Tri-sodium phosphate 0.10 The preparation of various pH solutions was carried out to add the appropriate amount of A solution to 50 ml B solution.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)